Article Contents
| Clin Exp Pediatr > Volume 66(7); 2023 |
|
Abstract
The gut covers a large surface area of the body and faces various external factors. The brain works in concert with commensal microbes in the gut to efficiently process the enormous amount of chemical signals that enter the gut every day. This review discusses: (1) evidence that gut bacteria can alter brain development and behavior, (2) mechanisms by which gut bacteria communicate with the brain, (3) preclinical and clinical studies demonstrating the impact of gut microbiota on autism spectrum disorder, and (4) variables worth consideration by future research on gut bacteria.
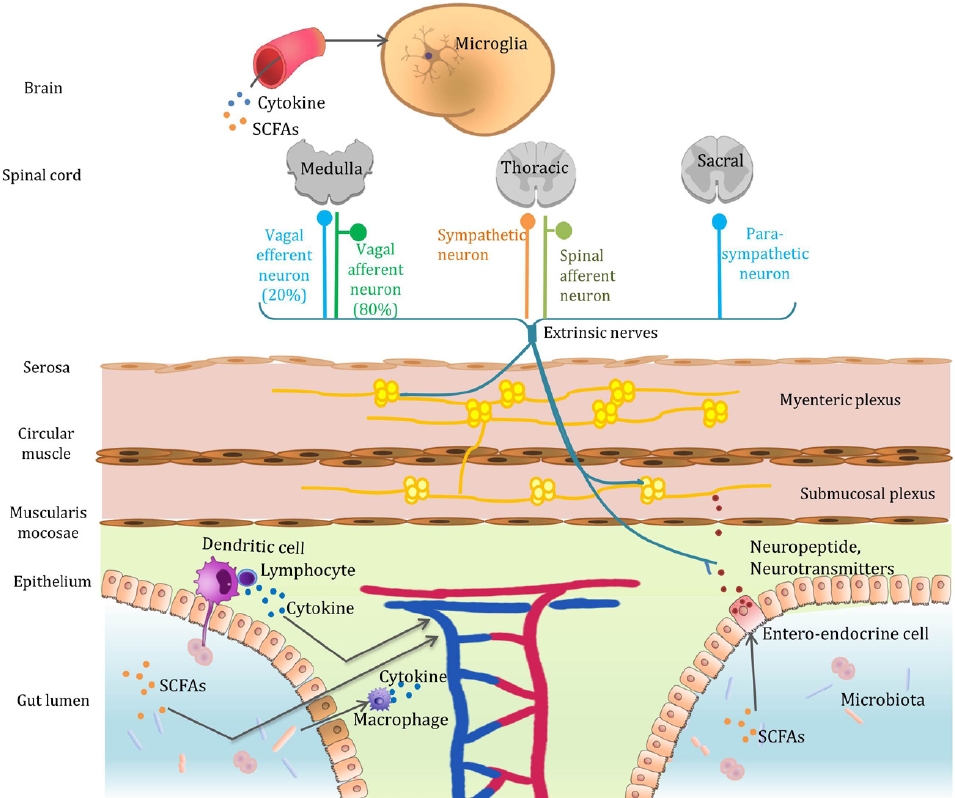
Graphical abstract. Microbiota-gut-brain axis. The mechanisms by which gut bacteria communicate with the brain include the secretion of neurotransmitters, neuromodulators, and proinflammatory cytokines; engaging the enteric nervous system and vagus nerve; and producing neuroactive metabolites. SCFA, short-chain fatty acid.
Except for the brain, which is equipped with a solid barrier, microbes cover all surfaces of the human body. Approximately 95% of human microbes live within the gastrointestinal tract. This is unsurprising as the surface area of the intestinal lumen corresponds to the size of 2 tennis courts (400 m2) [1]. According to recently revised estimates, there are nearly 38 trillion bacterial cells in the human body, mainly within the colon [2]. As they have lived in the human body for millions of years, there is reasonable doubt that they inevitably evolved with humans. Considerable research has revealed the impact of gut bacteria on their hosts. Gut bacteria detect various compounds that enter the body and notify the host in several ways. Furthermore, their presence affects the development of various organs, metabolic processes, and the immune system.
Meaningful studies recently examined the microbiota-gut-brain axis hypothesis to explain the effect of the gut microbiota on the brain. This review will focus on: (1) interesting evidence that gut bacteria can alter host development and behavior even in the brain, a mighty fortress; (2) mechanisms by which gut bacteria communicate with the brain; (3) preclinical and clinical studies that demonstrate the impact of the gut microbiota on autism spectrum disorder (ASD); and (4) variables worth consideration in future research on gut bacteria.
The blood-brain barrier (BBB) is impenetrable to all substances except for the limited nutrients the brain requires. Nevertheless, evidence suggests that the brain is influenced by the gut microbiota.
Germ-free (GF) mice exhibit increased adult hippocampal neurogenesis compared with control mice, an effect that occurs only in the dorsal hippocampus [3]. The dorsal hippocampus plays a critical role in spatial learning and memory. There is also a crucial early life period in which microbiota colonization affects adult hippocampal neurogenesis.
Second, genes related to myelination and myelin plasticity are upregulated in the prefrontal cortex of GF mice [4]. Recolonization with conventional microbiota could reverse these changes in the myelin as well as activity-related gene expression. The prefrontal cortex is affected by neuropsychiatric disorders, such as attention deficit hyperactivity disorder, ASD, depression, and schizophrenia. Therefore, the link between the gut bacteria and these diseases is worth investigating.
Third, the expression of synaptic plasticity-related genes is altered in GF mice [5]. Synaptophysin is a synaptic vesicle glycoprotein expressed by most neurons and neuroendocrine cells and an indirect marker of synaptic plasticity in the brain. The gut bacteria regulate synaptophysin expression and postsynaptic density-95, which is involved in excitatory synapse maturation.
Fourth, the gut microbiota is involved in the development of microglia, the primary immune cells in the brain. Microglia have many other functions in brain development including synaptic patterning, cell genesis, myelinogenesis, cell positioning, cell survival, axon dynamics, and cellular phagocytosis. Disturbances in the gut microbial community influence microglial development. GF or antibiotic-treated mice exhibit alterations in the microglial ratio and an immature phenotype [6]. However, defective microglia are restored by replenishing the gut microbiota and short-chain fatty acids (SCFAs), bacterial fermentation products containing acetic propionic acid, and butyric acid. Microglial density in cortical specimens was normalized after addition of the SCFA mixture to the drinking water of GF mice for 4 weeks. Moreover, by analyzing transcriptional results in GF mouse microglia, Matcovitch-Natan et al. [7] identified the dysregulation of dozens of genes involved in microglial development, despite maturity.
Knowing that disturbances in the gut microbiota can lead to abnormal neurogenesis, it may be possible to manipulate the gut microbiota in brain diseases accompanied by abnormal myelin formation, synapses, or microglia.
Gut microbial dysbiosis, which can occur at different stages of life, may contribute to the pathogenesis of various neuropsychiatric disorders and abnormal behaviors. The mechanisms by which gut bacteria communicate with the brain are as follows (Graphical abstract):
Functioning neurotransmitters and neuromodulators can be isolated from gut bacteria (Table 1) [8-24].
Monoamine neurotransmitters, such as dopamine, norepinephrine, and serotonin, can be derived from aromatic amino acids, such as phenylalanine, tyrosine, and tryptophan, by the action of aromatic amino acid decarboxylase within the gut bacteria. Gut bacterial species and enzymes involved in the metabolism of phenylalanine, tyrosine, and tryptophan were previously summarized by Liu et al. [25]. The altered expression of several neurotransmitters was observed in the central amygdala and dentate granule layer among the hippocampal subregions in GF mice [26]. GF mice also exhibit anxiolytic behavior and increased motor activity, and turnover rates of noradrenaline, dopamine, and 5-hydroxytryptamine are significantly higher in the striatum [5]. When GF mice are exposed to stress, anxious behaviors are more pronounced and the dopamine turnover rate in the upper brain involved in regulating stress and anxiety was significantly altered [27]. Some researchers have suggested that gut bacteria affect dopaminergic neurotransmission by modulating the mesocorticolimbic circuit [28].
Furthermore, the gut microbiota plays a critical role in central neurotrophin expression. Antibiotic-induced gut bacterial dysbiosis increases exploratory behavior and hippocampal brain-derived neurotrophic factor expression in mice [29]. This was reversed by normalizing the gut microbiota.
Gut microbes affect the brain by directly secreting neurotransmitters and neuromodulators that act on the body or regulate their expression.
Alterations in cytokine levels that accompany microbial infections may affect the developing brain. Inflammatory cytokines can promote the conversion of progenitor cells into dopaminergic neurons and decrease dendritic development [30-32]. Intrauterine exposure to specific gastrointestinal microbial pathogens can induce multiple psychopathologies, such as memory impairment or schizophrenia later in life [33-35]. The impact of maternal infection on fetal neurodevelopment is expected to vary with gestational age. For example, a maternal infection in the first trimester of pregnancy increases the risk of schizophrenia in the offspring [34].
Although the vagus nerve can perform both efferent and afferent roles, approximately 80% of nerve fibers are sensory organs that are mainly responsible for transmitting information about the state of the body organs to the brain. Postprandial satiety and sedation are produced partially by the active vagal afferent nerves in response to food intake. Likewise, the gut microbiota can signal the enteric nervous system and send signals to the brain via the vagus nerve [36]. Treatment with Lactobacillus rhamnosus reduced stress-induced corticosterone levels and anxiety- and depression-related behaviors in rats [37]. Notably, no neurochemical or behavioral effects were noted in mice after vagal nerve dissection, confirming the vagus nerve as the principal communication pathway between the gut bacteria and brain.
The gut microbiota can modulate host behavior via their metabolites. The parietal cells of the colon produce most of the serotonin in the periphery (60% in rats, 90% in humans). Serotonin production and secretion are affected by microbial metabolites including indole, SCFAs, secondary bile acids, α-tocopherol, p-aminobenzoate, and tyramine [23]. Furthermore, gut bacterial taxa and their metabolites differ between ASD and control mice [38]. When ASD mice are fed specific amino acid metabolites produced by bacterial fermentation (taurine, 5-aminovaleric acid), behavioral abnormalities (repetitive behavior and impaired social communication) significantly improve.
Moreover, gut microbes ferment polysaccharides to produce SCFAs (usually sodium butyrate). Butyrate-producing bacterial taxa are less abundant in children with ASD than in typically developing children [39]. Butyrate also strengthens the BBB by creating dense connections between neurons [40]. We previously questioned the association between the gut microbiota and neuropsychiatric disorders accompanying BBB permeability [41]. Evidence also suggests that gut bacterial metabolites play a role in hunger. Hunger can be modulated by glucagon-like peptide-1 secreted by colonic enteroendocrine L cells in response to the bacterial metabolite indole, which stimulates colonic vagal afferent activity in rats [42].
Many researchers have attempted to modify the gut microbiota in patients to treat various brain disorders. ASD is the most actively studied developmental disorder in this field. However, no results have clearly indicated a specific bacterial strain responsible for ASD, as shown in the meta-analyses below. Xu et al. [43] analyzed nine studies. They identified a lower abundance in the ASD groups in the Akkermansia, Bacteroides, Bifidobacterium, Escherichia coli, and Enterococcus genera and a greater abundance in the Faecilobacterium, Ruminococcus, and Lactobacillus genera. The analysis of Iglesias-Vázquez et al. [44] of 18 studies assessing 493 children with ASD and 404 controls reported a lower abundance in children with ASD in the Bifidobacterium and Coprococcus genera and a greater abundance in the genera Faecalibacterium, Bacteroides, Parabacteroides, Clostridium, and Phascolarctobacterium. AndreoMartínez et al. [45] analyzed 18 studies that assessed 642 patients and 356 controls. The Streptococcus and Bifidobacterium genera were less abundant in children with ASD. The included studies used different assessment methods, which could have been confounding factors. A recently published Korean study also reported inconsistent results: lower Bacteroides levels and higher Bifidobacterium levels in ASD patients versus controls [46].
Nevertheless, several clinical trials have attempted to alter the gut microbiota to treat patients with ASD (Table 2) [47-62]. Of them, microbial transfer therapy for children with ASD showed promising results with a steady improvement in core autism symptoms [47]. Moreover, the behavioral effects of the fecal microbiotal transplant persisted even at the 2-year follow-up [48]. However, behavioral outcomes have been inconsistent among studies using probiotics or prebiotics.
More clinical evidence is warranted to standardize treatments for manipulating the gut microbiota in the future. When planning a clinical study, i factors that can influence the gut microbiota must be recognized.
Clinical research on the gut microbiota is especially difficult because of the many possible variables that can change the outcome. However, it is difficult to control them simultaneously. As we analyzed earlier, the inconsistent results of many clinical studies on gut microbiota may be due to poor control of these confounding factors [63]. Many clinical studies have failed to incorporate these variables into their study design; moreover, whether they were unaware or deliberately ignored is unclear. Fig. 1 shows several known factors that can alter the gut microbiota, including diet, medicine, age, delivery mode, stress, and host factors [64-71]. For a successful clinical study, it is necessary to fully recognize and control these variables as much as possible.
The brain works in concert with commensal gut microbes to efficiently process the enormous amount of chemical signals that enter the gut daily. Elucidating the relationship between the gut microbiota and the brain has become essential to further our understanding of the brain’s development and behavior.
Fig. 1.
Factors affecting gut microbiota composition. AMP, antimicrobial peptide; IgA, immunoglobulin A; miRNA, microRNA.

Table 1.
Neuroactive amines and amino acids released by the gut bacteria
| Neurochemicals | Genus | References |
|---|---|---|
| Glutamate | Corynebacterium glutamycum, Lactobacillus plantarum, Lactobacillus paracasei, Lactococcus lactis | [8-10] |
| GABA | Escherichia coli, Pseudomonas, Lactococcus, Lactobacillus, Bifidobacterium | [11-15] |
| Dopamine | Escherichia, Bacillus, Lactococcus, Lactobacillus, Streptococcus | [16, 17] |
| Norepinephrine | Escherichia, Bacillus | [17] |
| Serotonin | Streptococcus, Escherichia, Enterococcus, Lactococcus, Lactobacillus, Corynebacterium | [16-18] |
| Histamine | Lactobacillus, Lactococcus, Streptococcus, Enterococcus | [19-21] |
| Acetylcholine | Lactobacillus, Bacillus | [22-24] |
Table 2.
Behavioral outcomes of clinical trials that engineered the gut microbiota in patients with autism spectrum disorder
| Intervention group | Study | Population (age, yr) | Intervention | Study design | Behavioral outcome |
|---|---|---|---|---|---|
| Probiotics alone or in combination | Sandler et al. (2000) [47] | 11 Regressive-onset ASD with antecedent antimicrobial use (3.5–7) | Vancomycin + (Lactobacillus acidophilus, Lactobacillus bulgaricus, Bifidobacterium bifidum) | Open-label trial | Improvements in ASD severity (↓ CARS), short-term |
| Parracho et al. (2010) [48] | 62 ASD (4–16) | Lactobacillus plantarum | RDBPC | Decreased disruptive, anti-social behavior, anxiety, and communication disturbances | |
| KałużnaCzaplińska (2012) [49] | 22 ASD with GI symptoms (4–10) | L. acidophilus | Open-label trial | Improvement in the ability to concentrate and carry out orders | |
| Arnold et al. (2019) [50] | 13 ASD with GI symptoms and anxiety (2–11) | 4 Lactobacillus strains + 3 Bifidobacterium strains + 1 Streptococcus strain | RDBPC, crossover | No significant behavioral change (PRASASD, ABC, SRS) | |
| Liu et al. (2019) [51] | 80 ASD (7–15) | L. plantarum | RDBPC | Improvement in some autism symptoms, primarily those associated with disruptive and rule-breaking behaviors and hyperactivity/impulsivity (more prominent in younger children) | |
| Niu et al. (2019) [52] | 114 ASD (ABA vs. ABA + probiotics) | 3 Bifidobacterium strains + 3 Lactobacillus strains | Open-label, 2-arm, randomized trial | Improvements in ASD severity (↓ ATEC) | |
| 40 HC (3–8) | |||||
| Santocchi et al. (2020) [53] | 85 ASD (mean, 4.2) | 4 Lactobacillus strains + 3 Bifidobacterium strains + 1 Streptococcus strain | RDBPC | Improvements in ASD severity (↓ ADOSCS) in the ASD without GI symptoms, although not significant in the ASD vs. the placebo | |
| Mensi et al. (2021) [54] | 131 ASD (mean, 86.1±41.1 mo) | L. Plantarum (105 ASD), others (26 ASD) | Open-label trial | Improvements in ASD severity (↓ CGI); Greater improvements in the Lact Plantarum group, no difference depending on the presence of GI symptom | |
| Shaaban et al. (2018) [55] | 30 ASD (5-9) | L. acidophilus + L. rhamnosus + B. longum and dried carrot | Open-label trial | Improvements in ASD severity (↓ ATEC) | |
| 30 HC children (5–9) | |||||
| Sanctuary et al. (2019) [56] | 8 ASD with GI symptoms (2–11) | B. infantis + bovine colostrum product | Randomized double-blind trial, crossover | Improved some atypical behaviors (irritability, stereotypies, hyperactivity, lethargy) | |
| Wang et al. (2020) [57] | 26 ASD (3–9) | B. infantis and lactis, L. rhamnosus and paracasei + fructooligosaccharide | RDBPC | Improvements in ASD severity (↓ ATEC) | |
| Prebiotics only | Grimaldi et al. (2018) [58] | 41 ASD (4–11) | Bimuno galactooligosaccharides | RDBPC | Improved only in anti-social behavior |
| Inoue et al. (2019) [59] | 13 ASD (4–9) | Partially hydrolyzed guar gum with β-endogalactomannase produced by a strain of Aspergillus niger | Open-label trial | Decreased behavioral irritability | |
| Fecal microbial transplant | Kang et al. (2017) [60] | 18 ASD with GI symptoms (7–16) | Bowel prep. with vancomycin + SHGM orally or rectally | Open-label trial | Improvements in ASD severity (↓ CARS, PGI-III, ABC, SRS) |
| Kang et al. (2019) [61] | 18 ASD with GI symptoms (7–17) | 2-Year follow-up after SHGM orally or rectally | Open-label trial | Only 17% were rated as severe ASD, 39% were in the mild to moderate, and 44% were below the ASD diagnostic cutoff scores. (83% of participants were severe ASD at the beginning of the trial.60)) | |
| Li et al. (2021) [62] | 40 ASD with GI symptoms (mean, 8.03±3.73) | Bowel prep. with polyethylene glycol + cFM orally or rectally | Open-label trial | Improved mood, behavior, emotion, language, and core ASD symptoms (↓ CARS, ABC, SRS) | |
| 16 HC (mean, 7.13±3.20) | Parent’s decreased anxiety levels |
ABA, applied behavioral analysis; ABC, Aberrant Behavior Checklist; ADOS-CS, Autism Diagnostic Observation Schedule-Calibrated Severity; ASD, autism spectrum disorder; ATEC, Autism Treatment Evaluation Checklist; CARS, Childhood Autism Rating Scale; cFM, fecal microbiota-filled capsules; CGI, clinical global impression; GI, gastrointestinal; HC, healthy controls; PGI-III, Parent Global Impressions-III; PRAS-ASD, Parent-Rated Anxiety Scale for ASD; Prep, preparation; RDBP, randomized double-blind placebo-controlled; SHGM, standardized human gut microbiota; SRS, Social Responsiveness Scale.
References
1. Peterson LW, Artis D. Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat Rev Immunol 2014;14:141–53.



2. Sender R, Fuchs S, Milo R. Revised estimates for the number of human and bacteria cells in the body. PLoS Biol 2016;14:e1002533.



3. Ogbonnaya ES, Clarke G, Shanahan F, Dinan TG, Cryan JF, O'Leary OF. Adult hippocampal neurogenesis is regulated by the microbiome. Biol Psychiatry 2015;78:e7. –9.


4. Hoban AE, Stilling RM, Ryan FJ, Shanahan F, Dinan TG, Claesson MJ, et al. Regulation of prefrontal cortex myelination by the microbiota. Transl Psychiatry 2016;6:e774.




5. Diaz Heijtz R, Wang S, Anuar F, Qian Y, Björkholm B, Samuelsson A, et al. Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci U S A 2011;108:3047–52.



6. Erny D, Hrabě de Angelis AL, Jaitin D, Wieghofer P, Staszewski O, David E, et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci 2015;18:965–77.




7. Matcovitch-Natan O, Winter DR, Giladi A, Vargas Aguilar S, Spinrad A, Sarrazin S, et al. Microglia development follows a stepwise program to regulate brain homeostasis. Science 2016;353:aad8670.


8. Tanous C, Chambellon E, Sepulchre AM, Yvon M. The gene encoding the glutamate dehydrogenase in Lactococcus lactis is part of a remnant Tn3 transposon carried by a large plasmid. J Bacteriol 2005;187:5019–22.




10. Zareian M, Ebrahimpour A, Bakar FA, Mohamed AKS, Forghani B, Ab-Kadir MSB, et al. A glutamic acid-producing lactic acid bacteria isolated from Malaysian fermented foods. Int J Mol Sci 2012;13:5482–97.



12. Chou HT, Kwon DH, Hegazy M, Lu CD. Transcriptome analysis of agmatine and putrescine catabolism in Pseudomonas aeruginosa PAO1. J Bacteriol 2008;190:1966–75.




13. Mazzoli R, Pessione E, Dufour M, Laroute V, Giuffrida MG, Giunta C, et al. Glutamate-induced metabolic changes in Lactococcus lactis NCDO 2118 during GABA production: combined transcriptomic and proteomic analysis. Amino Acids 2010;39:727–37.



14. Barrett E, Ross RP, O'Toole PW, Fitzgerald GF, Stanton C. γ-Aminobutyric acid production by culturable bacteria from the human intestine. J Appl Microbiol 2012;113:411–7.


15. Luck B, Horvath TD, Engevik KA, Ruan W, Haidacher SJ, Hoch KM, et al. Neurotransmitter profiles are altered in the gut and brain of mice mono-associated with Bifidobacterium dentium. Biomolecules 2021;11:1091.



16. Özogul F. Effects of specific lactic acid bacteria species on biogenic amine production by foodborne pathogen. Int J Food Sci Technol 2011;46:478–84.

17. Shishov VA, Kirovskaia TA, Kudrin VS, Oleskin AV. Amine neuromediators, their precursors, and oxidation products in the culture of Escherichia coli K-12. Prikl Biokhim Mikrobiol 2009;45:550–4.



18. Roshchina VV. Evolutionary considerations of neurotransmitters in microbial, plant, and animal cells. In: Lyte M, Freestone P, editors. Microbial endocrinology. New York: Springer, 2010:17–52.
19. Thomas CM, Hong T, van Pijkeren JP, Hemarajata P, Trinh DV, Hu W, et al. Histamine derived from probiotic Lactobacillus reuteri suppresses TNF via modulation of PKA and ERK signaling. PLoS One 2012;7:e31951.



20. Landete JM, De las Rivas B, Marcobal A, Muñoz R. Updated molecular knowledge about histamine biosynthesis by bacteria. Crit Rev Food Sci Nutr 2008;48:697–714.


21. Coton E, Rollan G, Bertrand A, Lonvaud-Funel A. Histamine-producing lactic acid bacteria in wines: early detection, frequency, and distribution. Am J Enol Vitic 1998;49:199–204.

22. Kawashima K, Misawa H, Moriwaki Y, Fujii YX, Fujii T, Horiuchi Y, et al. Ubiquitous expression of acetylcholine and its biological functions in life forms without nervous systems. Life Sci 2007;80:2206–9.


23. Girvin GT, Stevenson JW. Cell free choline acetylase from Lactobacillus plantarum. Can J Biochem Physiol 1954;32:131–46.


24. Horiuchi Y, Kimura R, Kato N, Fujii T, Seki M, Endo T, et al. Evolutional study on acetylcholine expression. Life Sci 2003;72:1745–56.


25. Liu Y, Hou Y, Wang G, Zheng X, Hao H. Gut microbial metabolites of aromatic amino acids as signals in host-microbe interplay. Trends Endocrinol Metab 2020;31:818–34.


26. Neufeld KM, Kang N, Bienenstock J, Foster JA. Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterol Motil 2011;23:255–64, e119.


27. Crumeyrolle-Arias M, Jaglin M, Bruneau A, Vancassel S, Cardona A, Daugé V, et al. Absence of the gut microbiota enhances anxiety-like behavior and neuroendocrine response to acute stress in rats. Psychoneuroendocrinology 2014;42:207–17.


28. González-Arancibia C, Urrutia-Piñones J, Illanes-González J, Martinez-Pinto J, Sotomayor-Zárate R, Julio-Pieper M, et al. Do your gut microbes affect your brain dopamine? Psychopharmacology (Berl) 2019;236:1611–22.



29. Bercik P, Denou E, Collins J, Jackson W, Lu J, Jury J, et al. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology 2011;141:599. –609. 609.e1-3.


30. Ling ZD, Potter ED, Lipton JW, Carvey PM. Differentiation of mesencephalic progenitor cells into dopaminergic neurons by cytokines. Exp Neurol 1998;149:411–23.


31. Marx CE, Jarskog LF, Lauder JM, Lieberman JA, Gilmore JH. Cytokine effects on cortical neuron MAP-2 immunoreactivity: implications for schizophrenia. Biol Psychiatry 2001;50:743–9.


32. Gilmore JH, Fredrik Jarskog L, Vadlamudi S, Lauder JM. Prenatal infection and risk for schizophrenia: IL-1beta, IL-6, and TNFalpha inhibit cortical neuron dendrite development. Neuropsychopharmacology 2004;29:1221–9.

33. Bilbo SD, Levkoff LH, Mahoney JH, Watkins LR, Rudy JW, Maier SF. Neonatal infection induces memory impairments following an immune challenge in adulthood. Behav Neurosci 2005;119:293–301.


34. Brown AS, Begg MD, Gravenstein S, Schaefer CA, Wyatt RJ, Bresnahan M, et al. Serologic evidence of prenatal influenza in the etiology of schizophrenia. Arch Gen Psychiatry 2004;61:774–80.


35. Sullivan R, Wilson DA, Feldon J, Yee BK, Meyer U, Richter-Levin G, et al. The International Society for Developmental Psychobiology annual meeting symposium: impact of early life experiences on brain and behavioral development. Dev Psychobiol 2006;48:583–602.



36. Thayer JF, Sternberg EM. Neural concomitants of immunity--focus on the vagus nerve. Neuroimage 2009;47:908–10.



37. Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci U S A 2011;108:16050–5.



38. Sharon G, Cruz NJ, Kang DW, Gandal MJ, Wang B, Kim YM, et al. Human gut microbiota from autism spectrum disorder promote behavioral symptoms in mice. Cell 2019;177:1600–18.e17.



39. Liu S, Li E, Sun Z, Fu D, Duan G, Jiang M, et al. Altered gut microbiota and short chain fatty acids in Chinese children with autism spectrum disorder. Sci Rep 2019;9:287.




40. Braniste V, Al-Asmakh M, Kowal C, Anuar F, Abbaspour A, Tóth M, et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci Transl Med 2014;6:263. ra158.



41. Weiss N, Miller F, Cazaubon S, Couraud PO. The blood-brain barrier in brain homeostasis and neurological diseases. Biochim Biophys Acta 2009;1788:842–57.


42. Fetissov SO. Role of the gut microbiota in host appetite control: bacterial growth to animal feeding behaviour. Nat Rev Endocrinol 2017;13:11–25.



43. Xu M, Xu X, Li J, Li F. Association between gut microbiota and autism spectrum disorder: a systematic review and meta-analysis. Front Psychiatry 2019;10:473.



44. Iglesias-Vázquez L, Van Ginkel Riba G, Arija V, Canals J. Composition of gut microbiota in children with autism spectrum disorder: a systematic review and meta-analysis. Nutrients 2020;12:792.



45. Andreo-Martínez P, Rubio-Aparicio M, Sánchez-Meca J, Veas A, Martínez-González AE. A meta-analysis of gut microbiota in children with autism. J Autism Dev Disord 2022;52:1374–87.



46. Ha S, Oh D, Lee S, Park J, Ahn J, Choi S, et al. Altered gut microbiota in Korean children with autism spectrum disorders. Nutrients 2021;13:3300.



47. Sandler RH, Finegold SM, Bolte ER, Buchanan CP, Maxwell AP, Väisänen ML, et al. Short-term benefit from oral vancomycin treatment of regressive-onset autism. J Child Neurol 2000;15:429–35.



48. Parracho HM, Gibson GR, Knott F, Bosscher D, Kleerebezem M, McCartney AL. A double-blind, placebo-controlled, crossover-designed probiotic feeding study in children diagnosed with autistic spectrum disorders. Int J Probiotics Prebiotics 2010;5:69–74.
49. Kałużna-Czaplińska J, Błaszczyk S. The level of arabinitol in autistic children after probiotic therapy. Nutrition 2012;28:124–6.


50. Arnold LE, Luna RA, Williams K, Chan J, Parker RA, Wu Q, et al. Probiotics for gastrointestinal symptoms and quality of life in autism: a placebo-controlled pilot trial. J Child Adolesc Psychopharmacol 2019;29:659–69.



51. Liu YW, Liong MT, Chung YE, Huang HY, Peng WS, Cheng YF, et al. Effects of Lactobacillus plantarum PS128 on children with autism spectrum disorder in Taiwan: a randomized, double-blind, placebo-controlled trial. Nutrients 2019;11:820.



52. Niu M, Li Q, Zhang J, Wen F, Dang W, Duan G, et al. Characterization of intestinal microbiota and probiotics treatment in children with autism spectrum disorders in China. Front Neurol 2019;10:1084.



53. Medical management of eating disorders – a practical handbook for healthcare professionals (2nd edition). Int J Health Care Qual Assur 2010;23(5): https://doi.org/10.1108/ijhcqa.2010.06223eae.002.

54. Mensi MM, Rogantini C, Marchesi M, Borgatti R, Chiappedi M. Lactobacillus plantarum PS128 and other probiotics in children and adolescents with autism spectrum disorder: a real-world experience. Nutrients 2021;13:2036.



55. Shaaban SY, El Gendy YG, Mehanna NS, El-Senousy WM, El-Feki HSA, Saad K, et al. The role of probiotics in children with autism spectrum disorder: A prospective, open-label study. Nutr Neurosci 2018;21:676–81.


56. Sanctuary MR, Kain JN, Chen SY, Kalanetra K, Lemay DG, Rose DR, et al. Pilot study of probiotic/colostrum supplementation on gut function in children with autism and gastrointestinal symptoms. PLoS One 2019;14:e0210064.



57. Wang Y, Li N, Yang JJ, Zhao DM, Chen B, Zhang GQ, et al. Probiotics and fructo-oligosaccharide intervention modulate the microbiotagut brain axis to improve autism spectrum reducing also the hyperserotonergic state and the dopamine metabolism disorder. Pharmacol Res 2020;157:104784.


58. Grimaldi R, Gibson GR, Vulevic J, Giallourou N, Castro-Mejía JL, Hansen LH, et al. A prebiotic intervention study in children with autism spectrum disorders (ASDs). Microbiome 2018;6:133.




59. Inoue R, Sakaue Y, Kawada Y, Tamaki R, Yasukawa Z, Ozeki M, et al. Dietary supplementation with partially hydrolyzed guar gum helps improve constipation and gut dysbiosis symptoms and behavioral irritability in children with autism spectrum disorder. J Clin Biochem Nutr 2019;64:217–23.



60. Kang DW, Adams JB, Gregory AC, Borody T, Chittick L, Fasano A, et al. Microbiota Transfer Therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: an open-label study. Microbiome 2017;5:10.




61. Kang DW, Adams JB, Coleman DM, Pollard EL, Maldonado J, McDonough-Means S, et al. Long-term benefit of microbiota transfer therapy on autism symptoms and gut microbiota. Sci Rep 2019;9:5821.




62. Li N, Chen H, Cheng Y, Xu F, Ruan G, Ying S, et al. Fecal microbiota transplantation relieves gastrointestinal and autism symptoms by improving the gut microbiota in an open-label study. Front Cell Infect Microbiol 2021;11:759435.



63. Lee K, Kim N, Shim JO, Kim GH. Gut bacterial dysbiosis in children with intractable epilepsy. J Clin Med 2020;10:5.



64. Bailey MT, Dowd SE, Galley JD, Hufnagle AR, Allen RG, Lyte M. Exposure to a social stressor alters the structure of the intestinal microbiota: implications for stressor-induced immunomodulation. Brain Behav Immun 2011;25:397–407.



65. Lyte M, Vulchanova L, Brown DR. Stress at the intestinal surface: catecholamines and mucosa-bacteria interactions. Cell Tissue Res 2011;343:23–32.



66. Claesson MJ, Jeffery IB, Conde S, Power SE, O'Connor EM, Cusack S, et al. Gut microbiota composition correlates with diet and health in the elderly. Nature 2012;488:178–84.



67. Zmora N, Suez J, Elinav E. You are what you eat: diet, health and the gut microbiota. Nat Rev Gastroenterol Hepatol 2019;16:35–56.



68. Jakobsson HE, Abrahamsson TR, Jenmalm MC, Harris K, Quince C, Jernberg C, et al. Decreased gut microbiota diversity, delayed Bacteroidetes colonisation and reduced Th1 responses in infants delivered by caesarean section. Gut 2014;63:559–66.


69. Yang C, Fujita Y, Ren Q, Ma M, Dong C, Hashimoto K. Bifidobacterium in the gut microbiota confer resilience to chronic social defeat stress in mice. Sci Rep 2017;7:45942.









 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link PubMed
PubMed Download Citation
Download Citation


