In order to prevent pneumococcal diseases in infants and toddlers, the 7-valent pneumococcal CRM197 protein conjugate vaccine (PCV7, Prevenar, Wyeth/Pfizer) has been used as an optional vaccination in Korea since November 2003
1). Since then, several vaccines containing additional serotypes have been developed and investigated clinically to expand the range of serotypes of
Streptococcus pneumoniae. Recently, two new pneumococcal protein conjugate vaccines 10-valent pneumococcal HiD-DiT protein conjugate vaccine (PCV10, Synflorix, GSK, Inc., Belgium) and 13-valent pneumococcal CRM197 protein conjugate vaccine (PCV13, Prevenar13, Pfizer, Inc. USA) were approved in foreign countries. PCV10 was approved first in Canada in December 2008 and then approved by the European Medicines Agency in March 2009. PCV13 was approved first in Chile in July 2009 and then approved in the USA in February 2010.
In Korea, two vaccines PCV10 and PCV13 were almost simultaneously approved by the Korean FDA in March 2010. This report summarizes guidelines for the use of these two newly introduced pneumococcal protein conjugate vaccines as suggested by the Committee on Infectious Diseases of the Korean Pediatric Society (
Box 1).
Pathogen
Streptococcus pneumoniae remains a leading cause of invasive infections including bacteremia and meningitis, as well as mucosal infections such as otitis media and pneumonia among children and adults.
Streptococcus pneumoniae is a gram-positive diplococci and is classified into around 90 serotypes according to the serological property of their capsular polysaccharide. In some of the serotypes, the polysaccharide has similar physicochemical properties and antigenicity. Most pneumococcal infections are caused by approximately 10 serotypes, and common serotypes differ according to the region, period, and age group
2).
In Korea,
Streptococcus pneumoniae is also believed to be the most common pathogen of bacterial diseases in children. A study analyzed the importance of
Streptococcus pneumoniae in invasive infections and clinical manifestations of pneumococcal diseases in Korean children who were hospitalized for invasive infections caused by one of 8 major bacterial pathogens at 18 university hospitals across the country during a 10 year period from 1996 to 2005
3). According to the results from this study, pneumococcal infections were responsible for 44% (123 cases) of the 279 cases of invasive bacterial diseases in immunocompetent children aged between 3 months to 5 years
3).
Serotypes 19F, 23F, 19A, 6B, 6A, 14, and 9V are common among the strains that were isolated from the nasopharynx and clinical specimens obtained from the children in Korea
4,
5). In addition, according to the results of analyzing the pattern of change in the serotype distribution during the period from 1991 to 2006 at a single center, while PCV7-serotypes decreased before the introduction of PCV7, PCV7-related serotypes such as 6A and 19A were on an increasing trend
6). The decrease in the proportion of PCV7-serotype was even more prominent in invasive strains isolated from children aged under 2 years and those under 5 years. According to the data reported recently by the Korean Centers for Disease Control and Prevention
7), among 74 invasive strains isolated from infants aged under 5 years during the period from 1996 to 2008, serotypes included in PCV7 were 59.5% (n=34), 3 serotypes added to both PCV10 and PCV13 such as serotype 1 (n=2), 5 (n=0) and 7F (n=0) were 2.7% (n=2), and serotypes added to PCV13 only such as 3 (n=1), 6A (n=8) and 19A (n=9) were 24.3% (n=18).
Vaccine components
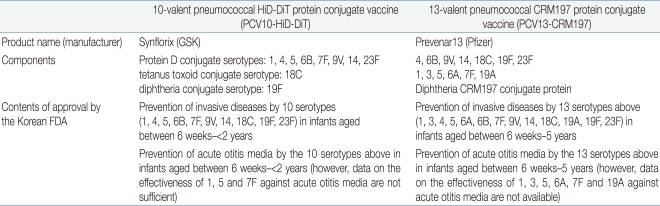
PCV10 contains 10 serotype polysaccharides (1, 4, 5, 6B, 7F, 9V, 14, 18C, 19F, and 23F) conjugated to 3 binding proteins; nontypeable
Haemophilus influenzae protein D (NTHi protein D), diphtheria toxoid, and tetanus toxoid. PCV10 contains 1 µg of polysaccharide from each serotype 1, 4, 5, 6B, 7F, 9V, 14 and 23F, and 3 µg from each serotype 4, 18C and 19F. 9-16 µg of NTHi protein D, 5-10 µg of tetanus toxoid, and 3-6 µg of diphtheria toxoid are also included in the vaccine. The vaccine contains aluminum as aluminum phosphate adjuvant, and no thimerosal preservative. It is produced in the form of a 0.5 mL pre-filled syringe (
Table 1).
PCV13 contains polysaccharides of 13 serotypes and diphtheria CRM197 protein. Six serotypes (1, 3, 5, 6A, 7F and 19A) are added to the 7 serotypes (4, 6B, 9V, 14, 18C, 19F and 23F) in PCV7, and all 13 serotype polysaccharides individually conjugated to CRM197. PCV13 contains approximately 2 µg of polysaccharide from each of 12 serotypes and 4 µg of polysaccharide from serotype 6B; the total concentration of CRM197 is 34 µg. The vaccine contains 0.125 mg of aluminum as aluminum phostphate adjuvant, and no thimerosal preservative. It is produced in the form of a 0.5 mL pre-filled syringe (
Table 1).
Immunogenicity
Newly developed pneumococcal conjugate vaccines may be approved without clinical trials on efficacy by comparing its immunogenicity with pre-existing licensed vaccines. The criteria for evaluating the immunogenicity of newly developed pneumococcal protein conjugate vaccines suggested by the WHO are as follows
8). The evaluation of immunogenicity after primary vaccinations during infancy uses IgG antibody titer specific to each serotype, and the criteria for non-inferiority are the percentage of subjects whose antibody titer for each serotype measured through WHO reference ELISA is ≥0.35 µg/mL and the geometric mean titer of serotype-specific IgG. However, if the association of antibody titer is proved sufficiently through bridge study between another antibody test method and WHO reference ELISA, the method may be used instead. For example, anti-capsular antibody titer 0.2 µg/mL measured by the antibody test method developed by GSK is equivalent to 0.35 µg/mL measured by WHO reference ELISA. As a secondary criterion, immunogenicity is evaluated by measuring functional antibody response through opsonophagocytosis assay (OPA). Then, after booster vaccination, the presence of anamnestic response is evaluated
9).
1. PCV10
Although the geometric mean concentration of anti-capsular IgG antibody was lower than that of PCV7, the WHO non-inferiority criteria was satisfied for 8 out of the 10 serotypes of PCV10 (5 out of the 7 PCV7-serotypes and 3 out of the newly added serotypes), but not for serotypes 6B and 23F. However, the OPA titer for these 2 serotypes was high, and the percentages of subjects with an OPA titer ≥1:8 was similar between PCV7 and PCV10 recipients. After the 4th booster dose, anti-capsular IgG antibody titers increased by 6-7 fold and OPA titers by 8-93 fold compared to those before vaccination
5).
2. PCV13
Among infants receiving the 3-dose primary infant series, 3 serotypes (6B, 9V, and 3) did not meet the primary endpoint criteria 1 month after the third dose, but all serotypes had detectable OPA antibodies and the percentages of subjects with an OPA antibody titer ≥1:8 were similar between PCV13 recipients (range: 90%-100%) and PCV7 recipients (range: 93%-100%). After the fourth dose, the non-inferiority criteria for anti-capsular IgG Ab geometric mean concentrations was met for 12 of the 13 serotypes; the non-inferiority criteria was not met for the response to serotype 3. Detectable OPA antibodies were present for all serotypes of the fourth dose; the percentage of PCV13 recipients with an OPA titer ≥1:8 ranged from 97%-100% for the 13 serotypes and was 98% for serotype 3
10).
The efficacy and effectiveness of the vaccines
No efficacy data is available for the two newly developed vaccines against invasive pneumococcal diseases, pneumonia, otitis media, and nasopharyngeal carriage. At the moment, the efficacy of PCV13 against pneumococcal diseases is predicted to be similar to the established efficacy and effectiveness of PCV7 in preventing invasive pneumococcal diseases, pneumonia, otitis media and reduction in nasopharyngeal carriers, and its preventive effectiveness is yet to be observed in the future.
Likewise, no research data has been reported on the efficacy and preventive effectiveness of PCV10. However, research has been done on the efficacy of 11-valent pneumococcal
HiD protein conjugate vaccine (PCV11) against otitis media and nasopharyngeal carriers
11). PCV11 is different from PCV10 in that it contains an additional serotype 3, and contains only one type of protein, NT
Hi protein D, that each serotype polysaccharide is conjugated to.
Safety
PCV10 and PCV13 are safe vaccines, comparable to safety of PCV7. Common adverse reactions include local responses such as pain, edema, and erythema in the injection site; systemic responses such as fever, irritability, and poor appetite; and rare adverse reactions such as convulsion, rash or itching.
Indications for Use (Table 1)
1. PCV10
1) Prevention of invasive diseases by 10 serotypes (1, 4, 5, 6B, 7F, 9V, 14, 18C, 19F, 23F) in infants aged between 6 weeks-<2 years
2) Prevention of acute otitis media by the 10 serotypes above in infants aged between 6 weeks-<2 years (however, data on the effectiveness of 1, 5 and 7F against acute otitis media are not sufficient)
2. PCV13
1) Prevention of invasive diseases by 13 serotypes (1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, 23F) in infants aged between 6 weeks-5 years (71 months)
2) Prevention of acute otitis media by the 13 serotypes above in infants aged between 6 weeks-5 years (71 months) (however, data on the effectiveness of 1, 3, 5, 6A, 7F and 19A against acute otitis media are not available).
Subjects and method of administration
1. Administration method
Both vaccines are administered intramuscularly as a 0.5 mL dose.
2. Subjects
(1) Infants aged between 6 weeks-6 months
i) Primary series: Administer 3 doses at ages 2, 4, and 6 months.
ii) Booster dose: The fourth dose is recommended at age 12-15 months and at least 6 months after the third dose.
(2) Infants aged 7 months or older who have not been completely vaccinated
i) Infants aged 7-11 months: Administer two doses with a minimum interval of one month. The 3rd dose is recommended at age ≥12 months with a minimum interval of 2 months from the 2nd dose.
ii) Infants aged 12-23 months: Administer two doses with a minimal interval of 2 months. It has not yet been established whether a supplemental dose is necessary after the 2nd dose.
2) PCV13
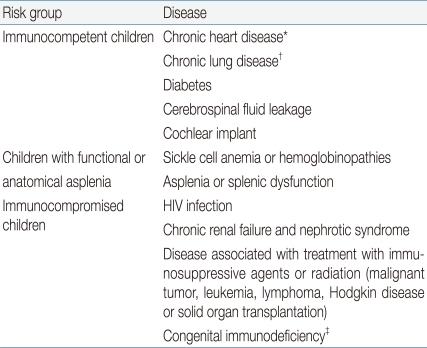
It is recommended to administer in infants aged between 2-59 months. In addition, vaccination is also recommended for all children aged 60-71months with underlying medical conditions that increase their risk for pneumococcal disease or complications (
Table 3)
12,
13).
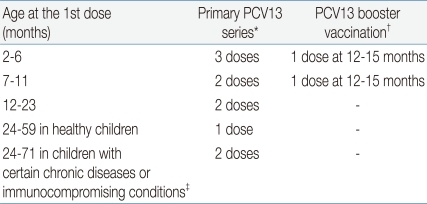
(1) No previous PCV7/PCV13 vaccination (Table 4)
For infants and toddlers aged 2-59 months, recommendations are the same as those previously published for PCV7.
1)
A. Infants aged 2-6 months
i) Primary series: Administer 3 doses at ages 2, 4, and 6 months at intervals of approximately 8 weeks. The first dose can be administered as early as 6 weeks, and the minimum interval is 4 weeks.
ii) Booster dose: The fourth dose is recommended at age 12-15 months and at least 8 weeks after the third dose.
B. Children aged ≥7 months
i) Infants aged 7-11 months: The first 2 doses should be administered with an interval of at least 4 weeks between doses. The third dose should be administered at age ≥12 months, at least 8 weeks after the second dose.
ii) Infants aged 12-23 months: Two doses are recommended, with an interval of at least 8 weeks between doses.
iii) Infants aged >24 months: Unvaccinated healthy children aged 24-59 months should receive a single supplemental dose. Unvaccinated children aged 24-71 months with underlying medical conditions increasing the risk for pneumococcal disease (
Table 3) should receive 2 doses with an interval of at least 8 weeks between doses.
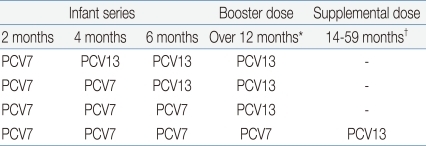
(2) Incomplete PCV7/PCV13 Vaccination (Table 5)
Infants and children who have received ≥ 1 dose of PCV7 should complete the vaccination series with PCV13.
A. Children aged <24 months
i) Children aged 12-23 months who have received 3 doses of PCV7 before age 12 months are recommended to receive 1 dose of PCV13, administered at least 8 weeks after the most recent dose of PCV7
ii) No additional PCV13 doses are recommended for children aged 12-23 months who received 2-3 doses of PCV7 before age 12 months and at least 1 dose of PCV13 at age ≥12 months.
B. Children aged ≥24 months
i) One dose of PCV13 is recommended for all healthy children aged 24-59 months with any incomplete PCV schedule.
ii) For children aged 24-71 months with underlying medical conditions who have received any incomplete schedule of <3 doses of PCV, 2 doses of PCV13 are recommended with intervals of at least 8 weeks. For those who have received 3 doses of PCV, a single dose of PCV13 is recommended.
(3) Complete PCV7 vaccination
i) A single supplemental dose of PCV13 is recommended for all children aged 14-59 months who have received 4 doses of PCV7 or another age-appropriate, complete PCV7 schedule.
ii) For children who have underlying medical conditions, a single supplemental PCV13 dose is recommended through 71 months. This includes children who have received 23-valent pneumococcal polysaccharide vaccine (PPSV23) previously.
iii) A supplemental dose should be administered at least 8 weeks after the most recent dose of PCV or PPSV23.
(4) Children aged 6-18 years in high-risk conditions
A single dose of PCV13 may be administered for children who have not received PCV13 previously and are at increased risk for invasive pneumococcal disease because of anatomic or functional asplenia, including sickle cell disease, immunocompromising conditions such as HIV infection, cochlear implant, or cerebrospinal fluid leaks, regardless of whether they have previously received PCV7 or PPSV23.
Interchangeability
As mentioned in the immunization schedule (
Table 5), it is possible to switch vaccines from previously administered PCV7 to PCV13. With regard to interchangeability between PCV7 and PCV10, there is a study that compared immunogenicity between two cases where the first had completed 3 doses of primary infant series with PCV7 following a 4th booster dose with PCV10, and another had completed both the primary infant series and booster dose with PCV10
9). Results showed that antibody titers for the 7 serotypes included in PCV7 increased sufficiently in both groups. However, there has not been any study comparing the immunogenicity of cross-administration between PCV7 and PCV10 for the primary infant series. So, at the moment, PCV10 after completion of primary infant series with PCV7 can be administered. However, it is not recommended to cross-administer PCV10 and PCV13 during primary infant series.
Contraindications
Follow the general contraindication guidelines for vaccination.





 PDF Links
PDF Links PubReader
PubReader PubMed
PubMed Download Citation
Download Citation


