Article Contents
| Korean J Pediatr > Volume 54(4); 2011 |
Abstract
Purpose
The purpose of this study was to evaluate the immune response to serotype 19A in children aged 12-23 months after immunization of the 19F containing 7-valent pneumococcal conjugate vaccine (PCV7).
Methods
Blood samples from a total of 45 subjects (age 12-23 months) were included in the study. Subjects were categorized according to immunization status into three groups as follows: 18 subjects with 3 primary doses and 1 booster dose of PCV7 (booster group), 21 subjects with 3 primary doses before 12 months of age (primary group), and 6 subjects with no vaccination history of PCV7 (control group). An ELISA and opsonophagocytic killing assay (OPKA) was done to evaluate the immune responses against serotypes 19F and 19A.
Results
According to the ELISA, all subjects had antibody titers ≥0.35 µg/mL for serotypes 19F and 19A in the booster and primary group and 83.0% and 66.7% in the control group, respectively. According to the OPKA, subjects with opsonic activity (≥20) against serotypes 19F and 19A were 100% and 61.1% of the subjects in the booster group and 66.7% and 19.0% in the primary group, respectively. No subjects in the control group had opsonic antibodies against both serotypes.
Streptococcus pneumoniae is an important bacterial pathogen which is a major cause of morbidity and mortality in children, the elderly and immunocompromised subjects of all ages. S. pneumoniae was the most common cause of invasive bacterial infections in children and it is a common cause of acute otitis media, sinusitis, and community-acquired pneumonia1). Moreover, S. pneumoniae is a leading cause for use of antimicrobial agents in clinical practice.
To reduce the burden of invasive pneumococcal diseases (IPD) and antimicrobial use, the 7-valent pneumococcal conjugate vaccine (PCV7, Prevenar®, Pfizer Inc, Philadelphia, PA, US) was developed and has been widely used in many countries. PCV7 was licensed in the US in February 2000, followed by Europe in 2001 and was introduced in Korea in November 2003. In the prevaccine era, the seven serotypes (4, 6B, 9V, 14, 18C, 19F and 23F) caused 80% of IPD in children in the US2) and 54% of IPD in Korea, according to an analysis of isolates from a single hospital-wide surveillance3).
After the introduction of PCV7, the overall IPD decreased by 77% and IPD due to vaccine serotypes decreased by 99% in children < 5 years age in the US2). In the development of the pneumococcal conjugate vaccine, serotype 19F was chosen as the representative of serogroup 19 in anticipation that 19F would induce a cross-reactive immune response against serotype 19A4). However, an increase in the proportion of IPD due to serotypes not included in the vaccine, especially 19A has been reported5, 6).
There are few reports regarding the cross-reactive immune response for serotype 19A after PCV7. In a previous report, antibodies against serotype 19A were elicited in healthy adult volunteers vaccinated with PCV77). However, in infants (7 months of age) vaccinated with 3 doses of PCV7, opsonic antibodies to 19A were detected in only 19% of the subjects8).
In this study, to determine the immune response of PCV7 against the vaccine-related serotype 19A in children, we evaluated the immune response to the vaccine serotype 19F and the vaccine-related serotype 19A in children aged 12-23 months of age. The immune response was compared among fully vaccinated subjects (3 primary doses and 1 booster dose), subjects only vaccinated with a primary series before 12 months of age (3 primary doses) and nonvaccinated subjects. Antibody titers against both serotypes were evaluated with the third-generation enzyme-linked immunosorbent assay (ELISA)9, 10), and to examine the functionality of these antibodies, an opsonophagocytic killing assay (OPKA) was performed.
A total of 45 subjects who visited Kangnam CHA Medical Center from September to December 2006 were included in this study. Study subjects were children aged 12-23 months who have been described previously11). Residual serum samples from children who had blood sampling for medical examination were obtained after informed consent. Subjects were categorized according to their immunization status against PCV7 (Prevenar®, Pfizer Inc, Philadelphia, PA, US) into three groups as follows: 18 subjects with 3 primary doses and 1 booster dose of PCV7 (booster group), 21 subjects with 3 primary doses before 12 months of age (primary group), and 6 subjects with no vaccination history of PCV7 (control group). Children with underlying immunodeficiency disorders or history of blood transfusion, immunoglobulin or systemic steroid medication were excluded from the study.
The study protocol was approved by the Institutional Review Board of Kangnam CHA Medical Center and was conducted in accordance with the Declaration of Helsinki. Immunization status against PCV7 was confirmed by immunization records.
Concentrations of antibodies against serotype 19F and 19A were determined by an ELISA using both C-polysaccharide and 22F serotype capsular polysaccharide absorption, as previously described9, 12). The ELISA was performed at the Center for Vaccine Evaluation and Study, Ewha Medical Research Institute at Ewha Womans University. In brief, the wells of a 96-well medium binding microtiter plate (Corning Inc., Corning, NY, US) were coated at 37℃ for 5 hours in a humidified chamber with 100 µL of capsular PS 19F or 19A (American Type Culture Collection [ATCC], Manassas, VA, US) diluted to a predetermined concentration. After being coated with the antigen, the plates were washed with 1 × Tris-buffered saline with 0.01% Brij 35 solution. All test sera were preabsorbed with C-polysaccharide (C-PS) (Statens Serum Institut, Copenhagen, Denmark) and 22F capsular PS (ATCC), and the reference standard 89-SF (provided by Carl Frasch, Center for Biological Evaluation and Review, Food and Drug Administration, Bethesda, MD, US) was preabsorbed with C-PS. Sera were serially diluted 2.5-fold in absorption solution and incubated at room temperature (RT) for 30 minutes. After incubation, samples were added to wells, titrated and incubated for 2 hours at RT. The wells in the plates were washed and incubated with alkaline phosphatase-conjugated goat antihuman IgG (Southern Biotech, Birmingham, AL, US). The amount of the enzyme in each well was determined with p-nitrophenyl phosphate powder (Sigma, St. Louis, MO, US) in diethanolamine buffer (Sigma, Yong-in city, Gyungi-do, Korea). The optical density was measured at 405 nm and the optical density of 690 nm was subtracted. Optical densities were converted to antibody concentrations using the CDC software for pneumococcal ELISA (written by Brian Plikaytis at the Centers for Disease Control and Prevention, Atlanta, GA, US). It can be downloaded free of charge from www.cdc.gov/ncidod/dbmd/bimb/elisa.htm). A detailed protocol can be found at www.vaccine.uab.edu.
The opsonic activities of the samples were evaluated using the OPKA as previously described13-16). In brief, target pneumococci were washed with opsonization buffer (Hanks' balanced salt solution with Mg/Ca, 0.1% gelatin, and 10% fetal bovine serum) by centrifugation, and diluted to the proper bacterial density. Target strains of serotype 19F and 19A were reported13, 14). Bacterial suspensions were pooled in equal volumes. Serum samples were incubated before serial dilutions in opsonization buffer at 56℃ for 30 minutes. Serum samples were diluted 5-fold before analysis due to limited amount of sera. Serially diluted serum (20 µL/well) was mixed with 10 µL of bacterial suspension in each well of round bottom 96-well plates (Corning Inc.). After a 30-minute incubation at RT with shaking (mini orbital shaker, Bellco Biotechnolgy, Vineland, NJ, US) at 700 rpm, 10 µL of 3-4 week old rabbit complement (PelFreeze Biologicals, Rogers, Arkansas, US) and 40 µL of differentiated HL60 cells (4×105 cells) were added to each well. HL60 cells were differentiated to granulocytes by culturing in RPMI-1640 with 10% fetal bovine serum and 1% L-glutamine and 0.8% dimethylformamide at a starting density of 4×105 cells/mL for 5 days. Plates were incubated in a tissue culture incubator at 37℃, 5% CO2 with shaking at 700 rpm. After 45-minute incubation, plates were placed on ice for 10-15 minutes and the final reaction mixture (10 µL) was spotted onto THY agar plates (Todd-Hewitt broth with 0.5% yeast extract and 1.5% agar). After the mixture was absorbed into the agar, an equal volume of overlay agar (THY with 0.75% agar and 25 mg/L of 2, 3, 5 -triphenyltetrazolium chloride) containing one of the antibiotics (spectinomycin or trimethoprim) was applied to each THY agar plate17). After an overnight incubation at 37℃, the number of bacterial colonies in the agar plates was enumerated. Opsonic indices were defined as the serum dilution that kills 50% of bacteria and were determined by linear interpolation. The lower limit of detection in the 5-fold serum dilution assay was 20. A detailed protocol is posted on a website (www.vaccine.uab.edu).
Anti-pneumococcal IgG antibodies and opsonic indices were transformed to a logarithmic scale and determined in geometric mean antibody concentrations (GMC) and geometric mean opsonic indices (GMI), respectively. Serum samples with opsonization indices of <20 were assigned a value of 10 for analysis purposes. The proportion of subjects of ≥0.35 µg/mL and opsonic indices ≥20 were determined, respectively. The χ2 test was used to compare proportions; this test was replaced by the Fisher's exact test when necessary. Antibody concentrations and opsonic indices were compared using the 2-tailed Student's t test.
The booster group consisted of 18 subjects. The mean age of the subjects was 18.8 months (range 16-23 months) and the mean interval between last vaccination and sampling was 3.3 months (range 1-7 months). The primary group consisted of 21 subjects and the mean age was 13.9 months (range 12-16 months). The mean interval between the last vaccination and sampling was 7.2 months (range 4-9 months). There were six subjects in the control group with a mean age of 14 months (range 12-16 months).
The GMCs in the booster group were higher for 19F than for 19A (10.68 µg/mL vs 5.75 µg/mL, P<0.05). The GMIs were also significantly higher for 19F compared with 19A (3,124 vs 172, P<0.05). All subjects reported antibody concentrations ≥0.35 µg/mL for both serotypes, whereas 100% and 61.1% showed opsonic activity for 19F and 19A, respectively (Table 1).
In the primary group, the GMCs did not differ between the two serotypes; however, the GMIs were higher for 19F compared with 19A (54 vs 15, P<0.05). Although all subjects had a titer of ≥0.35 µg/mL for both serotypes, only 66.7% and 19.0% of the subjects showed opsonic activity against 19F and 19A, respectively (Table 1).
There was no difference in GMCs and GMIs between the serotypes 19F and 19A. Subjects with antibody concentrations ≥0.35 µg/mL were 83.0% for 19F and 66.7% for 19A. However none of the subjects in this group showed functional opsonic activity against these two serotypes (Table 1).
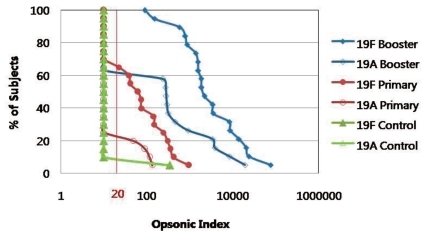
The GMCs and GMIs of 19F were higher in the booster group compared with the primary and control groups (P<0.05). For serotype 19A, the antibody concentration also increased according to vaccination status (control group< primary group< booster group, P<0.05). Although the GMIs showed no significant difference against serotype 19A between the control group and primary group, GMIs were significantly higher in the booster group compared with the primary group. Among subjects in the booster group, subjects with very high opsonic titers for serotype 19A were observed. Therefore, after vaccination with PCV7, although subtle cross-reactive immunity was noted against 19A in the primary group (19%), the response was augmented with a booster dose (61.1%); this cross-reactive immune response was significantly higher in the booster group (P<0.05) (Table 1, Fig. 1). This difference in immune response according to vaccination status can be seen well in the reverse cumulative distribution curve (Fig. 2). The graph shows that subjects who were vaccinated with a booster dose of PCV7 not only had a higher opsonic index for 19F, but also for 19A compared with those only vaccinated with the primary series. This is also seen in a comparison of the primary group and control group.
Another important finding is that when evaluating the immune response with the ELISA, subjects with a higher antibody titer for serotype 19F also showed high antibody titers for serotype 19A. Thus we can consider an immune response is elicited against serotype 19A after vaccination. However, according to the OPKA, many subjects with high opsonic indices against 19F showed nondetectable functional antibody for serotype 19A. Although all of the subjects in the primary and booster group had anti-19A IgG titers ≥0.35 µg/mL, only 61.1% and 19.0% had opsonic activity against serotype 19A, respectively. Therefore, among antibodies against serotype 19A detected by ELISA, a substantial proportion of these antibodies are nonfunctional antibodies which cannot be detected by OPKA.
In this study we evaluated the quantitative and qualitative immune responses against vaccine type 19F and vaccine-related 19A according to immunization status with PCV7 in children aged 12-23 months. We found minimal quantitative and qualitative immunity against serotypes 19F and 19A in nonvaccinated control subjects. However in subjects previously vaccinated with 3 doses of a primary series of PCV7, 66.7% showed opsonic activity against serotype 19F and 19.0% had opsonic activity against 19A at the age of 12-23 months. In addition, after 4 doses (including booster) of PCV7, 100% and 61.1% of the subjects had opsonic activity against serotypes 19F and 19A, respectively. From this we can speculate that after vaccination with PCV7, an immune response is elicited not only against vaccine type serotype 19F, but there is a weak cross-reactive immune response against serotype 19A after a primary series of 3 doses in infants and this response is amplified after a booster vaccination.
The importance of the booster dose has been recently documented18). In immunogenicity studies based on infants vaccinated with three primary doses of PCV7, opsonic antibodies against serotype 19A are reported in 3-19%8, 19). One study evaluated the opsonic response after three primary doses as well as after one booster dose of PCV7 in the same subjects20). In that particular study, although only 3.4% of the subjects showed opsonic activity after the primary series 27.6% had functional activity after the booster dose.
Clinical data has accumulated highlighting an increase in IPD due to serotype 19A2, 3, 5, 21). There are many theories regarding the increase of serotype 19A including vaccine-induced serotype replacement, secular trends, new clones and antibiotic pressure22). Reinert et al.22) speculated that highest rates of serotype 19A were found in countries with high antibiotic use (e.g., US, Spain and France) and countries with relatively low rates of antibiotic resistance (e.g., Norway and Germany) have a relatively stable incidence of disease due to 19A, despite the wide use of PCV7. Hausdorff et al.18) reported that the possibility of an increase in incidence of IPD due to 19A coincided with a shortage of PCV7 in the US between 2001 and 2004, resulting in many children receiving fewer than three primary doses and/or no booster dose. However in regions with high antibiotic resistance, disease due to 19A has increased. Whether PCV7 had an effect in lowering the slope of increase is currently unknown.
In this study the ELISA and OPKA were both performed to evaluate the immune response against PCV7 for a vaccine type serotype and vaccine-related serotype. However we found a substantial proportion of subjects with antibody titers ≥0.35 µg/mL with no detectable opsonic activity. This finding has been previously reported for serotype 19F. OPKA seropositivity (86%) was a better predictor of IPD efficacy than ELISA (99%). Therefore, some sera seropositive with ELISA are negative in the OPKA15). In this study, this discrepancy was more pronounced in the vaccine-related serotype compared with vaccine type serotype. Furthermore, more subjects of the primary or control group showed this compared with the booster group. OPKA correlates well with clinical effectiveness15). Therefore, evaluation of immune response using the OPKA would be more desirable, especially for determining the immune status in relatively weak immune responses such as those of infants, nonvaccinated or immunosuppression subjects and evaluation of vaccine-related serotypes.
This study has some limitations. Blood sampling was not done prospectively after vaccination, therefore there was a deviation in the interval from vaccination to blood sampling between subjects. Within each group, interval from vaccination ranged from 1-7 months in the booster group and 4-9 months in the primary group, however there was no definite correlation related with interval and OPA titer within each group. The WHO criteria for licensing pneumococcal vaccines recommends to evaluate immunogenicity 4 weeks after a primary series23). In regard, data presented in this study does not represent the immunogenicity to the PCV7, but rather gives us a mere insight towards the cross-reactive immune response. Also, samples were not obtained sequentially from the same subject after a primary and booster vaccination. Therefore difference in immune response among different subjects could be possible and this should be considered when interpreting the differences between the primary and booster results. There was no information regarding the subjects' previous pneumococcal disease history or other routes of acquisition for immunity to pneumococcus. Therefore there is possibility that the data does not solely reflect the effect of the vaccination. To compensate for this limitation, we included a group of nonvaccinated subjects as a control group, however there were a limited number of subjects in this group. Furthermore, in this study, due to a limited amount of sera, all samples were pre-diluted 5-fold, so the lower limit for opsonic activity was 20. The opsonic index which correlates with protection against IPD is 815, 19). Therefore the percentages of subjects with opsonic activity may be a under-estimation.
Protection against S. pneumoniae is based on serotype-specific antibodies. Hence, for a highly efficacious pneumococcal vaccine, it would be beneficial to include more serotypes. However this would lead to an increase in cost or possibly an increase in adverse events. Another method for enhancing coverage against pneumococcal serotypes would be to use different conjugate methods or carrier proteins in the formula leading to elicit different degrees of immunity including cross-reactive immunity for similar serotypes. The effect of a vaccine cannot be determined in a short time and many environmental and socio-economical factors should be considered. The results of this study give us a preliminary insight into the immune response for an important cross-reactive serotype 19A. However, continuous monitoring on the seroepidemiological changes of pneumococcus in clinical practices must coincide with the introduction of new vaccines.
In conclusion, in children 12-23 months age who were previously vaccinated with PCV7, a cross-reactive immune response is elicited in a small proportion of subjects against serotype 19A after a primary series of 3 doses and this response is amplified after booster vaccination.
Acknowledgment
This work was funded by a grant from the Kuhnil Academic Award, Korean Pediatric Society to K-H Kim. We thank MH Nahm for advisement in the OPKA. The authors state that they have no conflict of interest.
References
1. American Academy of Pediatrics. Pickering LK, Baker CJ, Kimberlin DW, Long SS,Pneumococcal infections. editors. Redbook: 2009 Report of the Committee in Infectious Diseases. 2009;28th ed. Elk Grove Village, IL: American Academy of Pediatrics, :524–535.
2. Centers for Disease Control and Prevention. Invasive pneumococcal disease in children 5 years after conjugate vaccine introduction--eight states, 1998-2005. MMWR Morb Mortal Wkly Rep 2008;57:144–148.


3. Choi EH, Kim SH, Eun BW, Kim SJ, Kim NH, Lee HJ, et al. Streptococcus pneumoniae serotype 19A in children, South Korea. Emerg Infect Dis 2008;14:275–281.



4. Jakobsen H, Sigurdsson VD, Sigurdardottir S, Schulz D, Jonsdottir I. Pneumococcal serotype 19F conjugate vaccine induces cross-protective immunity to serotype 19A in a murine pneumococcal pneumonia model. Infect Immun 2003;71:2956–2959.



5. Pelton SI, Huot H, Finkelstein JA, Bishop CJ, Hsu KK, Kellenberg J, et al. Emergence of 19A as virulent and multidrug resistant Pneumococcus in Massachusetts following universal immunization of infants with pneumococcal conjugate vaccine. Pediatr Infect Dis J 2007;26:468–472.


6. Whitney CG, Pilishvili T, Farley MM, Schaffner W, Craig AS, Lynfield R, et al. Effectiveness of seven-valent pneumococcal conjugate vaccine against invasive pneumococcal disease: a matched case-control study. Lancet 2006;368:1495–1502.


7. Lee HJ, Lim SY, Kim KH. Immune response to serotype 19A induced by serotype 19F in pneumococcal protein conjugate vaccine. Clin Microbiol Infect 2007;13:S294
8. Lee H, Nahm MH, Burton R, Kim KH. Immune response in infants to the heptavalent pneumococcal conjugate vaccine against vaccine-related serotypes 6A and 19A. Clin Vaccine Immunol 2009;16:376–381.



9. Concepcion NF, Frasch CE. Pneumococcal type 22f polysaccharide absorption improves the specificity of a pneumococcal-polysaccharide enzyme-linked immunosorbent assay. Clin Diagn Lab Immunol 2001;8:266–272.



10. Henckaerts I, Goldblatt D, Ashton L, Poolman J. Critical differences between pneumococcal polysaccharide enzyme-linked immunosorbent assays with and without 22F inhibition at low antibody concentrations in pediatric sera. Clin Vaccine Immunol 2006;13:356–360.



11. Park SE, Lee H, Lim SY, Kim KH. Immunogenicity of 7-valent pneumococcal conjugate vaccine related to booster immunization in Korean children. Korean J Pediatr 2008;51:622–628.

12. Wernette CM, Frasch CE, Madore D, Carlone G, Goldblatt D, Plikaytis B, et al. Enzyme-linked immunosorbent assay for quantitation of human antibodies to pneumococcal polysaccharides. Clin Diagn Lab Immunol 2003;10:514–519.



13. Burton RL, Nahm MH. Development and validation of a fourfold multiplexed opsonization assay (MOPA4) for pneumococcal antibodies. Clin Vaccine Immunol 2006;13:1004–1009.



14. Wang D, Burton RL, Nahm MH, Soong SJ. A four-parameter logistic model for estimating titers of functional multiplexed pneumococcal opsonophagocytic killing assay. J Biopharm Stat 2008;18:307–325.


15. Romero-Steiner S, Frasch CE, Carlone G, Fleck RA, Goldblatt D, Nahm MH. Use of opsonophagocytosis for serological evaluation of pneumococcal vaccines. Clin Vaccine Immunol 2006;13:165–169.



16. Romero-Steiner S, Libutti D, Pais LB, Dykes J, Anderson P, Whitin JC, et al. Standardization of an opsonophagocytic assay for the measurement of functional antibody activity against Streptococcus pneumoniae using differentiated HL-60 cells. Clin Diagn Lab Immunol 1997;4:415–422.



17. Kim KH, Yu J, Nahm MH. Efficiency of a pneumococcal opsonophagocytic killing assay improved by multiplexing and by coloring colonies. Clin Diagn Lab Immunol 2003;10:616–621.



18. Hausdorff WP, Hoet B, Schuerman L. Do pneumococcal conjugate vaccines provide any cross-protection against serotype 19A? BMC Pediatr 2010;10:4



19. Henckaerts I, Durant N, De Grave D, Schuerman L, Poolman J. Validation of a routine opsonophagocytosis assay to predict invasive pneumococcal disease efficacy of conjugate vaccine in children. Vaccine 2007;25:2518–2527.


20. Vesikari T, Wysocki J, Chevallier B, Karvonen A, Czajka H, Arsene JP, et al. Immunogenicity of the 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) compared to the licensed 7vCRM vaccine. Pediatr Infect Dis J 2009;28(4 Suppl): S66–S76.


21. Dagan R, Givon-Lavi N, Leibovitz E, Greenberg D, Porat N. Introduction and proliferation of multidrug-resistant Streptococcus pneumoniae serotype 19A clones that cause acute otitis media in an unvaccinated population. J Infect Dis 2009;199:776–785.


22. Reinert R, Jacobs MR, Kaplan SL. Pneumococcal disease caused by serotype 19A: review of the literature and implications for future vaccine development. Vaccine 2010;28:4249–4259.


23. Recommendations to assure the quality, safety and efficacy of pneumococcal conjugate vaccines. Proposed replacement of TRS 927, Annex 2. 2009. World Health Organization Available from: http://www.who.int/biologicals/areas/vaccines/pneumo/Pneumo_final_23APRIL_2010.pdf.
Fig. 1
Comparison of anti-19F IgG and anti-19A IgG measured by ELISA (A) and opsonophagocytic killing assay (B) in children immunized with primary and booster doses (Booster group ♦), primary doses (Primary group ●) of 7-valent pneumococcal conjugate vaccine and no vaccination (Control group ▲).
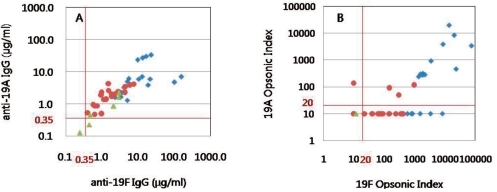
Fig. 2
Reverse cumulative distribution curves of opsonic indices against 19F and 19A according to vaccination status with 7-valent pneumococcal conjugate vaccine. Children immunized with primary and booster doses (Booster group ♦), primary doses (Primary group ●) of 7-valent pneumococcal conjugate vaccine and no vaccination (Control group ▲).




 PDF Links
PDF Links PubReader
PubReader PubMed
PubMed Download Citation
Download Citation


