Article Contents
| Korean J Pediatr > Volume 55(5); 2012 |
Abstract
Purpose
Dramatic improvement of hemangioma to propranolol has been recently reported; however, details on dose and duration of treatment, potential risks, and monitoring have not been determined. The objective of this study is to describe and analyze the use of propranolol as a first-line treatment or as a single therapy in management of complicated hemangioma.
Methods
A retrospective chart review of eight patients diagnosed with hemangioma and treated with propranolol in Kangbuk Samsung Hospital from February 2010 to April 2011 was performed.
Results
Eight patients with hemangioma with functional impairment, cosmetic disfigurement, or rapid growth were treated with propranolol. Five patients had solitary facial hemangioma. The mean age of symptoms at onset was 5 weeks. The median age for starting propranolol treatment was 5.5 months. Propranolol at 2 mg/kg/day was finally administered in divided doses with a gradual increase. Significant regression was observed in seven patients, and shrinkage in size, softening in consistency, and decrease in redness were evident within 4 weeks. Among them, six patients were still taking propranolol, and one patient had stopped after 12 months. Other one patient did not show significant improvement with satisfactory result after 3 months of propranolol use. Treatment with propranolol was well tolerated and had few side effects. No rebound growth was observed in any of the patients.
Infantile hemangiomas (IHs) are benign tumors of vascular endothelial cells and the most common tumors of infancy, present in 10% of live born white infants, with a lower incidence in children of Asian and African descent1). The frequency is higher in female infants, premature, low-birth weight babies, and those whose gestation is complicated by placental abnormalities2,3). IHs are characterized by rapid growth during the first year of life (proliferating phase) and a slow regression, which is usually completed at 7 to 10 years of age (involuting phase).
In most cases, due to the anticipated spontaneous regression of IH, there is no need for immediate therapy. There are inconsistencies across studies regarding the necessity of treatment, according to most studies, IHs require intervention during infancy because the lesion poses a threat to life or function or causes tissue distortion or destruction4). Current treatments include complete surgical excision, volume-reduction surgery, embolization therapy, sclerotherapy, pulsed dye laser treatment, cryosurgery, topical steroid application, intralesional corticosteroid therapy, high-dose systemic corticosteroid therapy, interferon, cyclophosphamide, or vincristine5). The mainstay of treatment is still systemic corticosteroids; however, oral propranolol has recently shown great promise as an effective systemic therapy.
In 2008, Leaute-Labreze et al.6) reported on treatment of severe hemangioma associated with cardiac complications with the non-selective ╬▓-blocker propranolol. Since 2008, several publications have reported a dramatic and rapid response to propranolol in treatment of hemangioma. However, according to our information, the benefits of propranolol therapy have been published only one case in Korean children with hemangioma7). Thus, we report on the clinical course in 8 cases of hemangioma managed with propranolol and the effect of propranolol in this study.
A retrospective chart review of eight patients diagnosed with hemangioma who were treated with propranolol from February 2010 to April 2011 was performed. Children visited the department of pediatrics at Kangbuk Samsung Hospital. We traced and comprehensively reviewed each patient's medical record from admission to discharge.
Demographic and clinical data included each patient's gender, gestational age, past medical history, the age when the lesion first appeared, location of the lesion, subtype of hemangioma, radiologic or histological examination, treatment indication, previous corticosteroid or other treatment, and clinical outcome. In addition, details regarding propranolol treatment, including dosage, age when first started and terminated, and side effects were obtained from the medical records, reviewed, and analyzed. For comparison, clinical photos were taken before, during, and after treatment. The treatment response was assessed changes of tumor size, consistency and color by serial photographs, medical records or radiological findings.
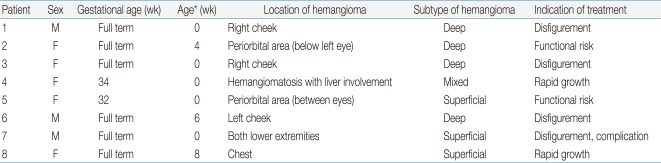
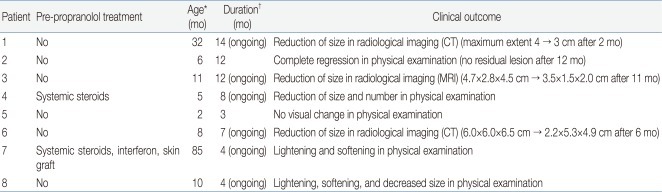
Eight children aged 9 months to 7 years (median, 16.5 months) were included in the study. Five children were females. Two children were born prematurely (33 weeks, 34 weeks). The mean age of symptom onset was 5 weeks (range, 0 to 20 weeks). Among the subjects, seven had solitary hemangioma, located around the cheek (3 patients), periorbital area (2 patients), chest (1 patient), and lower extremities (1 patient). The remaining patient had hemangiomatosis, hundreds of small, generalized hemangioma with hepatic involvement. None of our patients had medical contraindications for the use of propranolol. Patients' data are summarized in Table 1. The diagnosis of hemangioma was determined by the characteristic clinical appearance and radiologic findings. We ruled out differential diagnosis including non-involuting congenital hemangioma, congenital-onset tufted angioma, venous malformation (cavernous hemangioma) and lymphatic malformation (cavernous lymphangioma, cystic hygroma).
Five patients who had lesions located on the face had risks of functional impairment or significant cosmetic deformity. Two patients appeared to show rapid growth. Hemangioma of lower extremities was so large that it was likely to cause complication, including bleeding, ulceration, and infection or significant cosmetic deformity in the future.
One patient received treatment with systemic corticosteroid and interferon prior to oral propranolol therapy, but without any obvious clinical improvement. A patient of hemangiomatosis received systemic corticosteroid; however, no improvement was observed.
Propranolol treatments are summarized in Table 2. All patients were managed initially as inpatients and commenced on propranolol at 0.17 mg/kg/dose orally given at 8-hour intervals8). Vital signs and blood glucose were monitored 1 hour after each dose, corresponding with peak absorption time. If the first two doses were tolerated, the amount was doubled to 0.33 mg/kg/dose. After two more doses, propranolol was again doubled to 0.67 mg/kg/dose. This was the equivalent of 2.0 mg/kg/day. Patients were discharged after 48 to 72 hours on their final dosage of propranolol, and treatment continued at home.
The median age for starting propranolol treatment was 5.5 months (range, 2 months to 7 years). Shrinkage in size, softening in consistency, and decrease in redness were evident in all patients within 7 days. In seven patients, hemangioma continued to decrease in size or number or changed in color from a deep red to a blue or purple (Figs. 1, 2). Six patients were still taking propranolol for their hemangioma. In one patient, treatment was stopped after 12 months as a result of satisfactory clinical improvement; the lesion had softened and flattened (Fig. 3). One other patient did not show significant improvement for 3 months, and treatment was discontinued because she traveled abroad.
Patient 1 began treatment at 32 months of age, probably involuting phase, and showed an accelerated decrease in the size of the lesion.
Patient 6 had a swelling on the left cheek involving the left temple with extension into the left infero-auricular area and left posterolateral neck at admission. The lesion was noted at 1.5 months of age and showed rapid growth. Because that the lesion was deep and huge with normal overlying skin, computed tomography (CT) was performed for appropriate diagnosis. On CT reading, the lesion was most likely to be a vascular malformation. However, considering clinical history of the rapid growth, the possibility of hemangioma could not be excluded. Therefore, we tried using propranolol for treatment. Clinical improvement in signs and symptoms was seen within 2 weeks after starting treatment and the lesion was reduced in size from 6├Ś6├Ś6.5 cm to 2.2├Ś5.3├Ś4.9 cm within the first 6 months. The clinical course and response of propranolol treatment appeared to be compatible with hemangioma, and the diagnosis of hemangioma, rather than vascular malformation, was more likely.
Patient 7 had diffuse, superficial lesions on both extremities, which were unresponsive to corticosteroids and interferon. Therefore, propranolol treatment was started along with adjunctive laser therapy. Although evident regression was not yet observed, slight lightening and palpable softening of the lesion were observed within 7 days.
Since the serendipitous discovery of their efficacy in the 1960s, systemic corticosteroids have been the mainstay of therapy for treatment of hemangioma9,10). The regimen consists of administration of 2 to 4 mg/kg/day of prednisolone in a single morning dose, depending on the clinical situation. Corticosteroid treatments are most likely to be effective if given during the proliferative phase, typically during the first 1 to 4 months of life. Although steroids were effective, adverse effects of steroids are commonly observed. Side effects include gastrointestinal upset, irritability, weight gain, cushingoid appearance, hypertension, delayed growth, adrenal suppression, and immunosuppression11).
Propranolol is an attractive therapeutic alternative because its use can avoid the common adverse effects of prolonged high-dose steroid use and have dramatic and rapid response. Treatment with propranolol causes rapid halt of proliferation and promotes regression of problematic hemangioma with an apparent good safety profile, even after the growth phase is completed6). Propranolol is a non-selective beta adrenergic blocker, and has been used for several decades in treatment of hypertension, ischemic heart disease, arrhythmias, endocrine and neurologic disorders, and eye disorders. The mechanism of action of propranolol in hemangioma is not known; however, the proposed mechanism is that it induces vasoconstriction and capillary endothelial cell apoptosis. It also interferes with proangiogenic mechanisms involved in the growth phase12). The side effects of propranolol are well known and include transient bradycardia, hypotension, hypoglycemia, hyperkalemia, diarrhea and bronchospasm in patients with underlying reactive airways6,13-16).
As experience with propranolol becomes more extensive across multiple institutions, it may become the first line standard of therapy. However, so far, there has been no generally accepted agreement on the ideal treatment regimen with propranolol.
Doses of propranolol have generally been in the range of 1 to 3 mg/kg/day. Some cases have suggested that a good treatment response could be related to dose and made using the maximum safe dose (3 mg/kg/day)13). However, successful results from administration of doses ranging from 1 to 2 mg/kg/day have been reported8). Some studies have recommended utilizing gradual dose escalation and close monitoring during the first several days of treatment in order to minimize the risk of adverse effects14). For outpatients or infants younger than 3 months of age, slower increases in dosaging could be used. And infants younger than 6 months of age might need to be fed every 3 to 4 hours in order to avoid drug-induced hypoglycemia. We also treated with propranolol at the final dosage of 2 mg/kg/day with a gradually increasing dose and we found that this regimen was effective and safe.
The ideal duration of propranolol treatment has not yet been confirmed; however, one study appeared to demonstrate that treatment should be continued until the lesion is fully involuted or the child is 12 months of age and dose should be altered to account for the growth and weight gain of the child15). Another study suggested that propranolol should be gradually tapered over a period of 4 weeks17). The potential for more frequent relapse resulting from relatively shorter courses of treatment and rapid tail-off therapy has been demonstrated17). That is, re-growth occurred in a patient who received treatment for the shortest duration (2 months). In our study, mean or range of treatment duration has not yet been confirmed because most of the children are still taking propranolol for hemangioma; however, we will probably continue to treat at least until the age of spontaneous regression of the hemanigoma, depending on the clinical response.
Our patients tolerated propranolol well without significant side effects and recurrence. Some studies have reported that propranolol induced several adverse effects, including bradycardia, hypotension, and hypoglycemia16). However, they showed rapid recovery and no other serious toxic effects or complications have occurred. For patients with objective and continuous change in vital signs, a dose reduction or discontinuation of propranolol might be required. In addition, sweats, cold extremities, and diarrhea have been reported with propranolol in hemangioma18). These side effects might be associated with liquid formulations of oral propranolol, which contain various amounts of maltitol, propylene glycol, sorbitol, ethanol, and benzyl alcohol14).
We have not observed any relapse in our cases; however, several cases involving relapse of the hemangioma after early interruption of treatment or rapid process of tapering off have been reported17,19). These patients restarted propranolol treatment until the age of spontaneous regression of the hemangioma and showed good response to a second treatment course.
A few case studies have reported on the use of initial combined therapy of systemic corticosteroids and propranolol, which resulted in rapid resolution and allowed for quicker weaning of corticosteroids16). However, in many other cases, propranolol in management of hemangioma was found to be useful as the initial and single-agent therapy. According to one report, the effect of propranolol did not appear to be affected by previous medical treatments or history of surgery19). Further studies are needed in order to determine whether there is a benefit of propranolol treatment with combined therapy of corticosteroids or others.
Other beta-blockers, such as acebutolol, which has a differing beta-adrenoceptor affinity, have also been shown to be effective in treatment of hemangioma13). One study observed that a patient presenting with severe asthma as a noticeable side effect of propranolol was successfully treated with acebutolol as a substitute without relapse of the severe bronchoconstriction19). However, currently, because of a very good safety profile in children, propranolol has been the most widely reported beta-blocker agent used in treatment of hemangioma.
In conclusion, we observed that the use of propranolol was very effective in treatment of hemangioma without obvious adverse effects or relapse. However, due to the possible side effects of propranolol, a full cardiovascular and respiratory review prior to initiation of therapy and close monitoring during treatment with propranolol are needed. More clinical studies are required in order to determine the mechanisms of action and ideal treatment regimen.
References
1. Mulliken JB, Glowacki J. Hemangiomas and vascular malformations in infants and children: a classification based on endothelial characteristics. Plast Reconstr Surg 1982;69:412ŌĆō422.


2. Chiller KG, Passaro D, Frieden IJ. Hemangiomas of infancy: clinical characteristics, morphologic subtypes, and their relationship to race, ethnicity, and sex. Arch Dermatol 2002;138:1567ŌĆō1576.

3. Hemangioma Investigator Group. Haggstrom AN, Drolet BA, Baselga E, Chamlin SL, Garzon MC, et al. Prospective study of infantile hemangiomas: demographic, prenatal, and perinatal characteristics. J Pediatr 2007;150:291ŌĆō294.


4. Chang LC, Haggstrom AN, Drolet BA, Baselga E, Chamlin SL, Garzon MC, et al. Growth characteristics of infantile hemangiomas: implications for management. Pediatrics 2008;122:360ŌĆō367.


5. Pandey A, Gangopadhyay AN, Upadhyay VD. Evaluation and management of infantile hemangioma: an overview. Ostomy Wound Manage 2008;54:16ŌĆō18. 2022ŌĆō26. 28ŌĆō29.
6. Leaute-Labreze C, Dumas de la Roque E, Hubiche T, Boralevi F, Thambo JB, Taieb A. Propranolol for severe hemangiomas of infancy. N Engl J Med 2008;358:2649ŌĆō2651.


7. Lee EK, Choung HK, Kim NJ, Lee MJ, Kwon BS, Khwarg SI. A case of periorbital infantile capillary hemangioma treated with propranolol. J Korean Ophthalmol Soc 2010;51:1513ŌĆō1519.

8. Maguiness SM, Frieden IJ. Current management of infantile hemangiomas. Semin Cutan Med Surg 2010;29:106ŌĆō114.


9. Cohen SR, Wang CI. Steroid treatment of hemangioma of the head and neck in children. Ann Otol Rhinol Laryngol 1972;81:584ŌĆō590.


10. Rossler J, Wehl G, Niemeyer CM. Evaluating systemic prednisone therapy for proliferating haemangioma in infancy. Eur J Pediatr 2008;167:813ŌĆō815.


11. Lomenick JP, Reifschneider KL, Lucky AW, Adams D, Azizkhan RG, Woo JG, et al. Prevalence of adrenal insufficiency following systemic glucocorticoid therapy in infants with hemangiomas. Arch Dermatol 2009;145:262ŌĆō266.

12. Storch CH, Hoeger PH. Propranolol for infantile haemangiomas: insights into the molecular mechanisms of action. Br J Dermatol 2010;163:269ŌĆō274.


13. Holmes WJ, Mishra A, Gorst C, Liew SH. Propranolol as first-line treatment for rapidly proliferating infantile haemangiomas. J Plast Reconstr Aesthet Surg 2011;64:445ŌĆō451.


14. Lawley LP, Siegfried E, Todd JL. Propranolol treatment for hemangioma of infancy: risks and recommendations. Pediatr Dermatol 2009;26:610ŌĆō614.


15. Tan ST, Itinteang T, Leadbitter P. Low-dose propranolol for infantile haemangioma. J Plast Reconstr Aesthet Surg 2011;64:292ŌĆō299.


16. Rosbe KW, Suh KY, Meyer AK, Maguiness SM, Frieden IJ. Propranolol in the management of airway infantile hemangiomas. Arch Otolaryngol Head Neck Surg 2010;136:658ŌĆō665.


17. Chik KK, Luk CK, Chan HB, Tan HY. Use of propranolol in infantile haemangioma among Chinese children. Hong Kong Med J 2010;16:341ŌĆō346.

Fig.┬Ā1
Hemangioma in patient 3. Gross appearance at 11 months of age (A) and at 14 months of age (B). Magnetic resonance imaging at 3 months of age (C) and at 22 months of age (D). (A) Pretreatment. (B) After 3 month of propranolol treatment. (C) Before propranolol treatment, about 4.7├Ś2.8├Ś4.5 cm sized lobulated soft tissue mass in subcutaneous tissue of right buccomasseteric area. (D) After 11 month of propranolol treatment, decreased size (3.5├Ś1.5├Ś2.0 cm) of hemangioma.
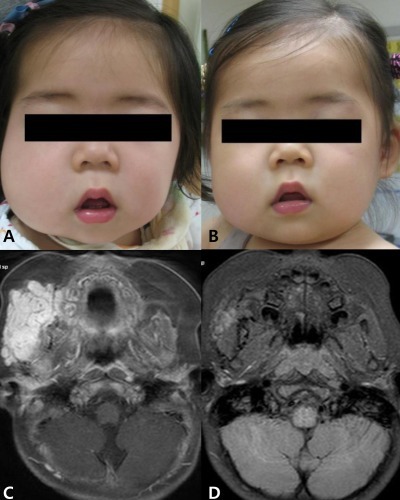
Fig.┬Ā2
Hemangioma in patient 4. Gross appearance at 5 months of age (A) and at 9 months of age (B). (A) Pretreatment. (B) After 4 months of propranolol treatment, decreased size and number of hemangioma.
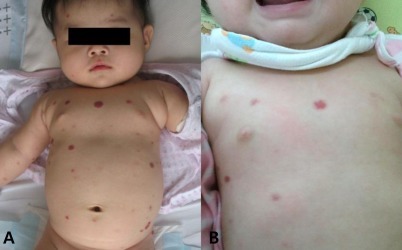
Fig.┬Ā3
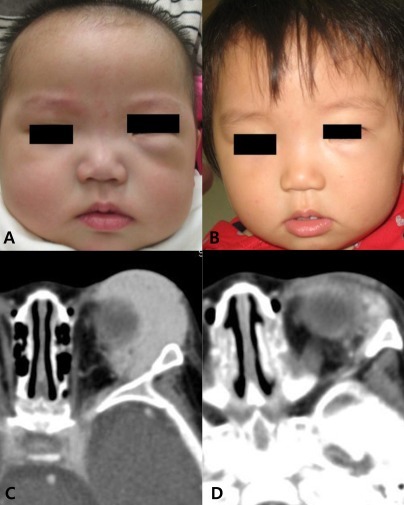
Hemangioma in patient 2. Gross appearance at 6 months of age (A) and at 18 months of age (B). Computed tomography at 6 months of age (C) and at 9 months of age (D). (A) Pretreatment. (B) After 12 months of propranolol treatment. (C) Before propranolol treatment, about 3.1├Ś1.3├Ś3.4 cm sized lobulated mass at extracornal area of inferolatreral aspect of left orbit. (D) After 3 months of propranolol treatment, decreased size (2.3├Ś0.8├Ś2.5 cm) of hemangioma.



 PDF Links
PDF Links PubReader
PubReader PubMed
PubMed Download Citation
Download Citation


