Article Contents
| Korean J Pediatr > Volume 57(5); 2014 |
|
Abstract
Purpose
A recent study analyzing several cytokines reported that long cardiopulmonary bypass (CPB) time and long aortic cross clamp (ACC) time were accompanied by enhanced postoperative inflammation, which contrasted with the modest influence of the degree of hypothermia. In this present study, we aimed to examine the effect of CPB temperature on the clinical outcome in infants undergoing repair of isolated ventricular septal defect (VSD).
Methods
Of the 212 infants with isolated VSD who underwent open heart surgery (OHS) between January 2001 and December 2010, 43 infants were enrolled. They were classified into 2 groups: group 1, infants undergoing hypothermic CPB (26Ōäā-28Ōäā; n=19) and group 2, infants undergoing near-normothermic CPB (34Ōäā-36Ōäā; n=24).
Results
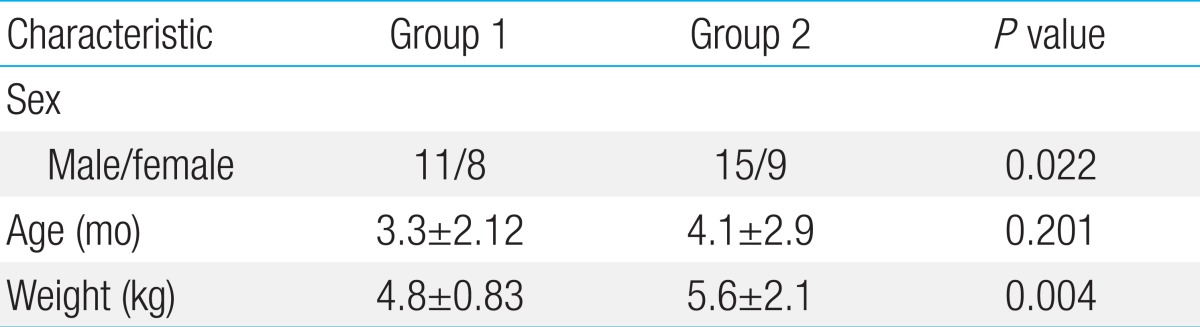
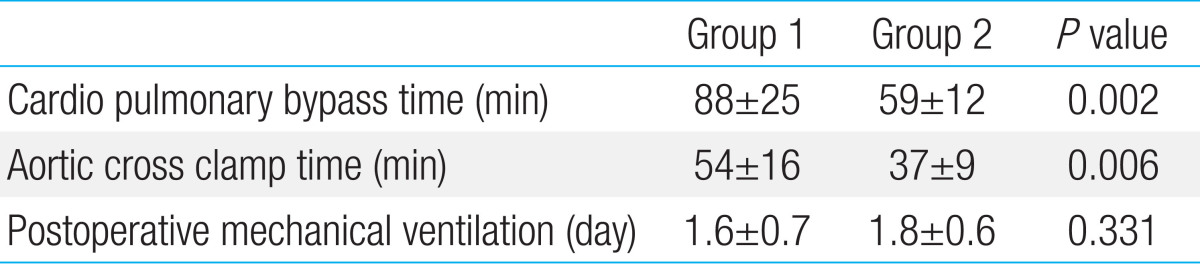
The age at the time of the OHS, and number of infants aged<3 months showed no significant differences between the groups. The CPB time and ACC time in group 1 were longer than those in group 2 (88 minutes vs. 59 minutes, P=0.002, and 54 minutes vs. 37 minutes, P=0.006 respectively). The duration of postoperative mechanical ventilation was 1.6 days in group 1 and 1.8 days in group 2. None of the infants showed postoperative neurological and developmental abnormalities. Moreover, no postoperative differences in the white blood cell count and C-reactive protein levels were noted between two groups.
Cardiac operations involving cardiopulmonary bypass (CPB) can induce a systemic inflammatory response involving activated neutrophils, a disturbed balance between proinflammatory and anti-inflammatory cytokines, with interactions among many other mediators such as arachidonic acid derivatives, products from oxidative stress, nitric oxide, endothelin-1 and platelet activating factor1).
In many centers performing pediatric open heart surgery (OHS), the use of hypothermia is common. The main aim of cooling the body is to protect major organs from ischemic injury by reducing the oxygen consumption and whole body inflammatory response to CPB2,3).
A recent study analyzing several cytokine demonstrated that long CPB time and long aortic cross clamp (ACC) time were accompanied by enhanced postoperative inflammation in contrast to the modest influence of the degree of hypothermia4).
This study evaluated through laboratory findings whether hypothermia during OHS is related to inflammation in infants undergoing OHS.
Between January 2001 and December 2010, 621 infants underwent OHS in Kyungpook National University Medical Center. Among them, 212 patients were VSD patients. The lowest bypass temperature of hypothermia (26Ōäā-29Ōäā) and near-normothermia (34Ōäā-36Ōäā) were selected3) (Fig. 1). Sixty-five infants were in two temperature groups. Twenty-two patients with insufficient laboratory data and records were excluded. Forty-three infants were enrolled and they were classified into 2 groups: group 1, infants undergoing hypothermic CPB (26Ōäā-29Ōäā) and group 2, infants undergoing near-normothermic CPB (34Ōäā-36Ōäā).
Values are expressed as the mean┬▒standard deviation. Univariate comparisons of continuous variables were conducted using the unpaired Student t test. Univariate analyses of the differences in proportion between the two groups were accomplished using the chi-square analysis. Differences with a P value of <0.05 were considered to be statistically significant. To examine changes over time after surgery, mixed model analysis were employed using PASW statistics 18.0 (SPSS Inc., Chicago, IL, USA).
Among of them, 19 infants were group 1 and 24 were group 2. Age on OHS, and number of patients below 3 months old showed no significant differences between groups 1 and 2 (Table 1). Patients' body weight was significant different between groups 1 and 2 (P=0.004). In group 1, 4 infants were Down syndrome and 8 infants had admission for respiratory infection before OHS. There were no patients with pneumonia or sepsis at the time of surgery. In group 2, number of infants with Down syndrome and pre-OHS admission were 2 and 3, respectively. The duration of postoperative mechanical ventilation was 1.6 days in group1 and 1.8 days in group 2.
Priming volume showed no differences between two groups. The CPB time and ACC time in group 1 were longer than those in group 2 (P=0.002 and P=0.006) (Table 2). In both groups, postoperative WBC and CRP increased after the surgery and decreased gradually. But there was no difference between two groups. (Figs. 2 and 3; P value of WBC difference 0.27, and P value of CRP difference 0.29)
There were no infants with postoperative seizure, prolonged low Glasgow Coma Scale score and developmental abnormalities.
VSD is the most commonly recognized congenital heart defect and one of the most common defects requiring surgical closure4). Surgical closure of isolated VSD is known to be safe and effective4,5). CPB has been widely utilized in the surgical correction of VSD for decades6).
CPB activates blood cells, such as endothelial cells, neutrophils, platelets and stimulates the cytokines such as tumor necrosis factor-a, interleukin (IL) 1, IL-6, and IL-87,8). These series of responses induce inflammatory reaction and these factors induce complications like bleeding, thromboembolism, and organ dysfunction9,10).
Various treatment strategies have been tested to reduce the systemic inflammation induced by CPB. Medical treatment includes corticosteroids. In the several adult studies, hypothermia during the surgery is known to protect major organs from ischemic injury by reducing the oxygen consumption, the complement activation, and whole body inflammatory response to CPB11,12,13).
The recent studies have shown that hypothermia does not offer significant advantages over near-normothermia during pediatric CPB. Stocker et al.3) studied the influence of CPB temperature of pediatric patients. Mild hypothermia group showed similar clinical outcomes and inflammatory markers with hypothermia group. Eggum et al.4) evaluated the inflammatory response in children with mild or moderate hypothermia. Clinical postoperative outcomes were similar in two groups.
Long CPB time tends to increase plasma cytokine levels. Long ACC time also increases cytokine levels and inflammatory responses4). In our study, hypothermia group had longer CPB and ACC times. Even though hypothermia decreased inflammatory reaction, longer CPB and ACC time may resulted in similar clinical and laboratory outcomes in both groups. Overall in our study, neonates and infants requiring correction of simple VSD, hypothermic and near-normothermic CPB could have similar clinical outcome. And there was no laboratory differences suggesting inflammatory reaction between two groups.
This study has limitations. This study was designed under the retrospective chart review and could only include WBC count and CRP as inflammatory markers. Cytokines could not be included. And patients with diverse age, different type of congenital heart disease needs to be investigated.
Inflammation and organ damage is a concern after the OHS. In this study, near-normothermic CPB showed similar clinical outcome and inflammatory response compared to hypothermic patients. Near-normothermic bypass should be concerned in pediatric OHS.
References
1. Paparella D, Yau TM, Young E. Cardiopulmonary bypass induced inflammation: pathophysiology and treatment. An update. Eur J Cardiothorac Surg 2002;21:232ŌĆō244.



2. Corno AF, von Segesser LK. Is hypothermia necessary in pediatric cardiac surgery? Eur J Cardiothorac Surg 1999;15:110ŌĆō111.


3. Stocker CF, Shekerdemian LS, Horton SB, Lee KJ, Eyres R, D'Udekem Y, et al. The influence of bypass temperature on the systemic inflammatory response and organ injury after pediatric open surgery: a randomized trial. J Thorac Cardiovasc Surg 2011;142:174ŌĆō180.


4. Eggum R, Ueland T, Mollnes TE, Videm V, Aukrust P, Fiane AE, et al. Effect of perfusion temperature on the inflammatory response during pediatric cardiac surgery. Ann Thorac Surg 2008;85:611ŌĆō617.


5. Scully BB, Morales DL, Zafar F, McKenzie ED, Fraser CD Jr, Heinle JS. Current expectations for surgical repair of isolated ventricular septal defects. Ann Thorac Surg 2010;89:544ŌĆō549.


6. Kogon B, Butler H, Kirshbom P, Kanter K, McConnell M. Closure of symptomatic ventricular septal defects: how early is too early? Pediatr Cardiol 2008;29:36ŌĆō39.


7. Mahle WT, Lundine K, Kanter KR, Forbess JM, Kirshbom P, Tosone SR, et al. The short term effects of cardiopulmonary bypass on neurologic function in children and young adults. Eur J Cardiothorac Surg 2004;26:920ŌĆō925.


8. H├Čvels-G├╝rich HH, Schumacher K, Vazquez-Jimenez JF, Qing M, Huffmeier U, Buding B, et al. Cytokine balance in infants undergoing cardiac operation. Ann Thorac Surg 2002;73:601ŌĆō608.


9. Beghetti M, Rimensberger PC, Kalangos A, Habre W, Gervaix A. Kinetics of procalcitonin, interleukin 6 and C-reactive protein after cardiopulmonary-bypass in children. Cardiol Young 2003;13:161ŌĆō167.


10. Allan CK, Newburger JW, McGrath E, Elder J, Psoinos C, Laussen PC, et al. The relationship between inflammatory activation and clinical outcome after infant cardiopulmonary bypass. Anesth Analg 2010;111:1244ŌĆō1251.


11. Rossaint J, Berger C, Van Aken H, Scheld HH, Zahn PK, Rukosujew A, et al. Cardiopulmonary bypass during cardiac surgery modulates systemic inflammation by affecting different steps of the leukocyte recruitment cascade. PLoS One 2012;7:e45738



Fig.┬Ā1
Flow diagram for the classification of infants. OHS, open heart surgery; VSD, ventricular septal defect.
Fig.┬Ā2
Preoperative and postoperative (day 1, 2, and 4) white blood cell (WBC) count of both groups. The differences between the 2 groups were not statistically significant (P=0.27). POD, postoperative day.
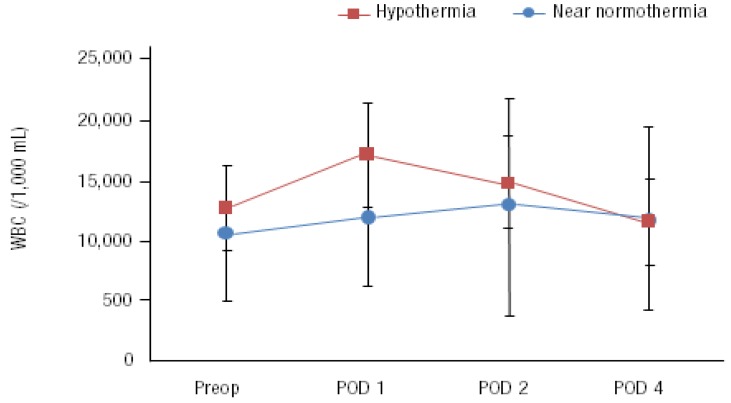
Fig.┬Ā3
Preoperative and postoperative (day 1,2, and 4) C-reactive protein (CRP) level of both groups. The differences between the 2 groups were not statistically significant (P=0.29).




 PDF Links
PDF Links PubReader
PubReader PubMed
PubMed Download Citation
Download Citation


