Article Contents
| Korean J Pediatr > Volume 58(8); 2015 |
|
Abstract
Purpose
It is difficult to differentiate between central precocious puberty (CPP) and premature thelarche (PT) in girls. The aim of this study was to investigate the diagnostic usefulness of pelvic ultrasonography to distinguish between CPP and PT in girls with early breast development.
Methods
This study included girls with early breast development who visited the clinic between January 2012 and December 2013. Clinical, laboratory, and pelvic ultrasonographic data were evaluated. CPP and PT were confirmed using the gonadotropin-releasing hormone stimulation test.
Results
A total of 248 girls aged 7-8 years were included, among whom 186 (75.0%) had CPP and 62 (25.0%) had PT. The uterine length, transverse diameter, fundus, volume, and cross-sectional area were significantly larger in the CPP group (uterine length, 2.45±0.50 cm vs. 2.63±0.49 cm, P=0.015; uterine volume, 0.95±0.62 cm3 vs. 1.35±0.76 cm3, P<0.001). However, there were no differences in the fundus/cervix ratio and ovarian measurements. In receiver operating characteristic analysis, a uterine volume of at least 1.07 cm3 was the most predictive parameter for CPP with an area under the curve of 0.670 (95% confidence interval, 0.593-0.747).
Conclusion
Uterine measurements by pelvic ultrasonography in girls with early pubertal development were significantly larger in the CPP group. However, the diagnostic value of ultrasonographic parameters was not high because of a considerable overlap of values between the two groups. Therefore, pelvic ultrasonography in combination with clinical and laboratory tests may be useful to distinguish between CPP and PT in girls.
Precocious puberty is defined as the appearance of the secondary sexual characteristics before the age of 8 years in girls1). Central precocious puberty (CPP) is caused by the premature activation of the hypothalamic gonadotropin-releasing hormone (GnRH) pulse generator and is generally idiopathic2). CPP can be associated with diverse problems such as compromised final adult height and psychological as well as emotional conflicts3,4,5). Therefore, an early diagnosis and a proper management are critical6,7).
However, it is difficult to differentiate between CPP and premature thelarche (PT). PT is featured by an isolated appearance of breast development, that is not progressive and does not require treatment8). The differentiation between CPP and PT is confirmed by clinical, radiologic and laboratory tests such as physical examination, evaluation of bone age, height velocity measurement and GnRH stimulation test1). The laboratory determination of the peak luteinizing hormone (LH) concentration during the GnRH stimulation test is considered the most important diagnostic process, although it had some disadvantages including time-consuming multiple samples resulting in discomfort to patients and a low sensitivity despite of its high specificity9).
The transabdominal pelvic ultrasonography has been used to differentiate CPP from PT10). Pelvic ultrasonography is noninvasive and relatively less time-consuming. Several studies reported that larger uterine and ovarian measurements were associated with CPP than PT11,12,13,14). However, it is not conclusive to the diagnostic role of pelvic ultrasonography in patients with early pubertal signs.
The aim of this study was to investigate the diagnostic usefulness of pelvic ultrasonography to differentiate between CPP and PT in girls with early breast development and to determine the optimal cutoff values of ultrasonographic measurements to distinguish CPP and PT.
Girls with early breast development who were referred for the evaluation of CPP to our pediatric endocrinology clinic between January 2012 and December 2013 were included in this study. Inclusion criteria were as follows: (1) chronological age between 7 and 8 years at the first visit; (2) breast budding before the age of 8 years; (3) breasts with Tanner stage 2 or more on the first examination in our clinic; (4) advanced bone age by one or more years; and (5) GnRH stimulation test and pelvic ultrasonography for the evaluation of CPP. Exclusion criteria were as follows: (1) peripheral precocious puberty; (2) CPP due to an organic intracranial lesion; (3) presence of a chronic illness such as diabetes mellitus and thyroid disorders; and (4) medication which may affect the hypothalamic-pituitary-ovarian axis. Of 250 girls who met the inclusion criteria, 248 were enrolled in the study. Two girls were excluded for following reasons: one girl presented with CPP after the treatment of anaplastic astrocytoma and the other girl had a previous history of acute lymphocytic leukemia.
The retrospective review of medical records for this study was approved by the Institutional Review Board of the Inje University Ilsan Paik Hospital (IB-1407-029). We reviewed the medical records of the subjects who met the inclusion criteria. Demographic and clinical parameters around the day of GnRH stimulation test and pelvic ultrasonography were investigated including chronological age, bone age, height, body weight, body mass index (BMI), sexual maturity rate, parental height and hormonal profiles. The standard deviation score (SDS) of height, body weight and BMI for the same age and sex were calculated using the LMS methods proposed in 2007 Korean National Growth Charts15). The assessment of the bone age was performed using the Greulich-Pyle method16).
A standard GnRH stimulation test was conducted in the early morning after overnight fasting. Basal serum samples were obtained for the measurement of LH, follicular stimulating hormone (FSH) and estradiol just before the intravenous bolus injection of 100 µg of GnRH (Relefact, Sanofi-Aventis, Frankfurt, Germany). After the administration of GnRH, blood samples for the determination of LH and FSH concentration were withdrawn at 30, 45, 60, and 90 minutes. Serum LH, FSH and estradiol level were measured by an electrochemiluminescence immunoassay (Roche Diagnostics GmbH, Manheim, Germany). Within-run and total precision of the hormonal assays were ranging from 0.7% to 1.2% and from 1.6% to 2.2% for LH; from 2.5% to 2.8% and from 3.6% to 4.5% for FSH; from 1.7% to 3.3% and 2.2% to 4.7% for estradiol. The limits of detection were 0.1 IU/L for LH, 0.1 IU/L for FSH and 5.0 pg/mL for estradiol. A peak LH concentration of at least 5 IU/L during GnRH stimulation test was considered as CPP7). Subjects with a peak LH concentration of less than 5 IU/L were classified as PT.
Transabdominal pelvic ultrasonography was obtained with a micro convex probe 8C (3.5-11.5 MHz, Logiq 9, GE healthcare, Milwaukee, WI, USA). Ultrasonography was performed with full bladder filling by a single experienced radiologist (Y.S.K.). Following parameters of the uterus were evaluated including the length, transverse diameter, fundal anteroposterior diameter (fundus), cervical anteroposterior diameter (cervix) and the presence of endometrial echogenicity. The uterine cross-sectional area was calculated by multiplying the length by the fundal anteroposterior diameter. The uterine volume was calculated according to the ellipse formula (length×transverse diameter×fundal anteroposterior diameter×0.5233). The ratio of the fundal to cervical anteroposterior diameter (fundus/cervix ratio) was calculated. In ovaries, the height, width, and length were evaluated. The circumference of each ovary was calculated according to the ellipse circumference formula {2.222×[(height)2+(length)2]1/2}. The volume of each ovary was computed using the same ellipse formula as that for uterus.
Statistical analyses were performed with STATA 12.1 (StataCorp LP., College Station, TX, USA). Results were expressed as mean±standard deviation. Student t test was used to compare demographic and clinical parameters between the CPP and PT group. Logistic regression analysis was performed to determine the association between the results of GnRH stimulation test and clinical, laboratory and ultrasonographic variables. Receiver operating characteristic (ROC) analyses was used to investigate the predictive ability of laboratory and ultrasonographic parameters17). The optimal cutoff values were determined using the Youden index (J), which is defined by "J= maximum (sensitivity+specificity-1)"18). Sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) was calculated for each cutoff value. A P value less than 0.05 was considered significant.
Of 248 subjects, 186 (75.0%) were diagnosed with CPP and 62 (25.0%) with PT (Table 1). The chronological age was 8.36±0.44 years in the CPP group and 8.28±0.68 years in the PT group (P=0.367). The bone age was 10.06±0.77 years in the CPP group and 10.06±0.72 years in the PT group (P=0.886). The advancement of bone age over chronological age was 1.67±0.66 years in the CPP group and 1.77±0.60 years in the PT group (P=0.306). The BMI SDS was significantly higher in the PT group, although the height SDS showed no significant difference between the two groups. Laboratory parameters during GnRH stimulation test including basal LH, peak LH, basal FSH, peak FSH, basal LH/FSH ratio, peak LH/FSH ratio, estradiol, insulin-like growth factor-I (IGF-I), and IGF-I SDS were significantly higher in the CPP group (Table 1).
Measurements of uterine length, uterine transverse diameter, fundus, uterine volume and uterine cross-sectional area were significantly higher in the CPP group (Table 2). However, the fundus/cervix ratio was not different between both groups. An endometrial echogenicity was observed in one subject with CPP. There was no significant difference in both ovarian circumferences and volumes between both groups (Fig. 1).
Univariate logistic regression analysis was carried out to determine variables affecting the diagnosis of precocious puberty during GnRH stimulation test. Basal LH, basal FSH, basal LH to FSH ratio, IGF-I SDS and BMI SDS were significant parameters. In ultrasonographic findings were uterine length, uterine transverse diameter, fundus, uterine volume and uterine cross-sectional area predictors of the diagnosis of CPP (Table 3).
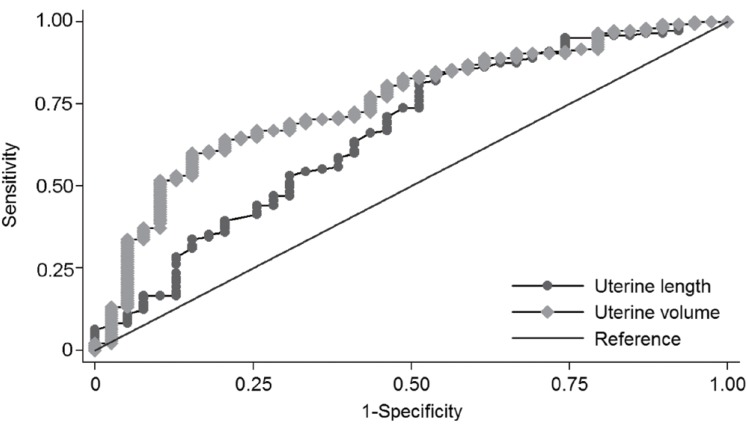
ROC curves were constructed based on logistic regression analyses. The area under the curve (AUC) with 95% confidence iterval (CI) for basal LH, basal FSH and basal LH/FSH ratio was 0.766 (0.708-0.825), 0.727 (0.652-0.802), and 0.769 (0.712-0.825), respectively. There was no significant difference between parameters (P=0.329). The AUC (95% CI) for uterine length, uterine transverse diameter, fundus, uterine volume and uterine cross-sectional area was 0.588 (0.503-0.673), 0.656 (0.577-0.734), 0.660 (0.579-0.741), 0.670 (0.593-0.747), and 0.661 (0.586-0.737), respectively (Fig. 2). The AUC of uterine volume was the biggest among ultrasonographic parameters (P=0.04). The optimal cutoff value for each parameter was selected using the Youden index (J) based on ROC analyses. Sensitivity, specificity, PPV, and NPV for each cutoff value are shown in Table 4.
Further analyses were performed according to the BMI. All subjects were grouped according to their BMI assigned into two categories, either the obese group (BMI at least 85th percentile for age and sex) or the normal weight group (BMI less than 85th percentile for age and sex). Pelvic ultrasonographic findings were analyzed between both groups. In the normal weight group, there were significantly greater values in the uterine length, uterine transverse diameter, fundus, uterine volume and the uterine cross-sectional area, although the fundus/cervix ratio, ovarian circumferences and volumes were not significantly different. In ROC analysis, the AUC for uterine length was 0.663 (95% CI, 0.561-0.765) with a cutoff value of 2.2 cm. The AUC for uterine volume was the largest with 0.751 (95% CI, 0.666-0.835) and was statistically significant (P=0.002) (Fig. 3). The optimal cutoff of uterine volume by the Youden index (J) was 1.09 cm3 with a sensitivity of 60.0% and a specificity of 84.6%. In the obese group, there were no differences in pelvic ultrasonographic findings between the CPP and PT group.
In girls with early pubertal signs, the GnRH stimulation test was considered the gold standard for the laboratory confirmation of CPP. However, GnRH stimulation test had several drawbacks such as the discomfort to patients, a time-consuming procedure with multiple samples, relatively high costs and a low sensitivity despite high specificity9). The pelvic ultrasonography is a useful method to evaluate pelvic organs. It is noninvasive, relatively less time-consuming and relative inexpensive19).
In this study, we compared the pelvic ultrasonographic parameters in the CPP and PT group. Significant differences were observed in uterine length, uterine transverse diameter, fundus, uterine volume and uterine cross-sectional area volume. Those measurements were larger in the CPP group. However, the fundus/cervix ratio showed no difference (Table 2). In the consensus statement, uterine length and uterine volume were suggested as useful parameters to distinguish CPP from PT. The cutoff values for uterine length and uterine volume ranged from 3.4 to 4.0 cm and from 1.0 to 3.0 cm3, respectively7). Haber et al.12) reported a 100% sensitivity and specificity for the cutoff value of 1.8 mL of uterine volume. De Vries et al.11) reported a sensitivity of 88.8% and specificity of 89.4% for the cutoff value of 2.0-mL uterine volume and a sensitivity of 80.2% and specificity of 57.8% for the cutoff value of 3.4-cm uterine length. In another report, the diagnostic cutoff value was 3.74-cm uterine length and 3.48-mL uterine volume20). In our study, the cutoff value of uterine length was 2.2 cm and 1.07 cm3 for uterine volume. These were smaller than those reported in other reports (Table 4). The sensitivity and specificity at each cutoff point was 83.3% and 33.9% for uterine length and 59.1% and 71.0% for uterine volume. In the ROC analysis, the AUC of uterine length and volume was 0.588 (95% CI, 0.503-0.673) and 0.670 (95% CI, 0.593-0.747), indicating low accuracy for a diagnostic test (Fig. 2)21). In the previous article reported on Korean girls, uterine measurements were similar to our study13). Reasons for the differences of cutoff values may be ethnic differences, body size differences, interpersonal variations of radiologists and performance differences among ultrasonographic machines.
The fundus/cervix ratio was reported as an important parameter of the pubertal uterus. The prepubertal uterus had a tubular shape and the fundus/cervix ratio was almost 122,23). In puberty, hormonal influences to the uterus made the fundus prominent with a fundus/cervix ratio greater than 1. Previous studies reported a bigger fundus/cervix ratio in the CPP group11,20). However, in other reports there were no significant differences in the fundus/cervix ratio between the CPP and PT group13,24). In this study, the fundus/cervix ratio was 1.49±0.46 in the CPP group and 1.50±0.59 in the PT group, without significant differences.
Ovarian measurements and morphology were other parameters to differentiate between CPP and PT. In previous studies, the average ovarian volume and ovarian area were larger in the CPP group11,13,14,20,25). An ovarian circumference with a suggested cutoff of at least 4.5 cm is a good indicator for the pubertal development11). However, in this study, there was no significant difference in all ovarian measurements (Table 2).
A subgroup analysis was performed in the obese and normal weight group. In the normal weight group, uterine measurements were larger in the CPP group, except for the fundus/cervix ratio. The optimal cut off was 2.2 cm for the uterine length and 1.09 cm3 for the uterine volume. In the ROC analysis, the AUC of uterine length and volume were 0.663 (95% CI, 0.561-0.765) and 0.751 (95% CI, 0.666-0.835), indicating a moderate accuracy (Fig. 3)21). In the obese group, there was no significant difference in uterine and ovarian measurements between the CPP and PT group. It could be suggested that the uterine growth could be influenced by the body fat. However, more research is required on this topic.
The uterine endometrial echogenicity may be of help in the diagnosis of CPP, although it was highly specific, but less sensitive11,26). In our study, endometrial echogenicity was observed in only one case with advanced CPP, suggesting that the evaluation in this study was performed in the early phase of puberty. The color Doppler during the pelvic ultrasonography showed a lower uterine arterial impedance in CPP patients27). However, no color Doppler was carried out in this study.
This study has several limitations. The study design was retrospective. Control subjects were not included without pubertal development. During the pelvic ultrasonography, color Doppler was not performed and uterine and ovarian morphology were not described. However, a large number of subjects with suspicious precocious puberty were enrolled and the pelvic ultrasonographic variance was minimized because subjects were in a relatively narrow range of age. Also only one experienced radiologist performed all imaging studies.
In conclusion, uterine measurements in the pelvic ultrasonography of girls with early pubertal development were significantly larger in the CPP group with laboratory confirmation after GnRH stimulation test in this study. The uterine volume of at least 1.07 cm3 was the most predictive parameter among pelvic ultrasonographic findings to diagnose CPP. Pelvic ultrasonography was more efficient to differentiate CPP from PT in the normal weight group than in the obese group. However, the diagnostic value of ultrasonographic parameters was not high because of a considerable overlap between values (Fig. 1). Therefore, the pelvic ultrasonography with an adjunct to clinical and laboratory parameters is helpful to enhance the diagnostic precision between CPP and PT. Additionally reference values of pelvic ultrasonographic parameters among Korean girls according to chronological age, bone age and pubertal stage is needed.
Conflicts of interest
Conflict of interest:
No potential conflict of interest relevant to this article was reported.
References
1. Lee PA. Central precocious puberty. An overview of diagnosis, treatment, and outcome. Endocrinol Metab Clin North Am 1999;28:901–918. xi


2. Parent AS, Teilmann G, Juul A, Skakkebaek NE, Toppari J, Bourguignon JP. The timing of normal puberty and the age limits of sexual precocity: variations around the world, secular trends, and changes after migration. Endocr Rev 2003;24:668–693.


3. Brauner R, Adan L, Malandry F, Zantleifer D. Adult height in girls with idiopathic true precocious puberty. J Clin Endocrinol Metab 1994;79:415–420.


4. Yang JH, Han SW, Yeom CW, Park YJ, Choi WS, Seo JY, et al. Depression and self-concept in girls with perception of pubertal onset. Ann Pediatr Endocrinol Metab 2013;18:135–140.



5. Mrug S, Elliott M, Gilliland MJ, Grunbaum JA, Tortolero SR, Cuccaro P, et al. Positive parenting and early puberty in girls: protective effects against aggressive behavior. Arch Pediatr Adolesc Med 2008;162:781–786.


7. Carel JC, Eugster EA, Rogol A, Ghizzoni L, Palmert MR, et al. ESPE-LWPES GnRH Analogs Consensus Conference Group. Consensus statement on the use of gonadotropin-releasing hormone analogs in children. Pediatrics 2009;123:e752–e762.


8. Salardi S, Cacciari E, Mainetti B, Mazzanti L, Pirazzoli P. Outcome of premature thelarche: relation to puberty and final height. Arch Dis Child 1998;79:173–174.



9. Pescovitz OH, Hench KD, Barnes KM, Loriaux DL, Cutler GB Jr. Premature thelarche and central precocious puberty: the relationship between clinical presentation and the gonadotropin response to luteinizing hormone-releasing hormone. J Clin Endocrinol Metab 1988;67:474–479.


10. Shawker TH, Comite F, Rieth KG, Dwyer AJ, Cutler GB Jr, Loriaux DL. Ultrasound evaluation of female isosexual precocious puberty. J Ultrasound Med 1984;3:309–316.


11. de Vries L, Horev G, Schwartz M, Phillip M. Ultrasonographic and clinical parameters for early differentiation between precocious puberty and premature thelarche. Eur J Endocrinol 2006;154:891–898.


12. Haber HP, Wollmann HA, Ranke MB. Pelvic ultrasonography: early differentiation between isolated premature thelarche and central precocious puberty. Eur J Pediatr 1995;154:182–186.


13. Kang HJ, Nam JS, Cho WK, Cho KS, Park SH, Jung MH, et al. Pelvic ultrasonography findings in girls with precocious puberty. J Korean Soc Pediatr Endocrinol 2010;15:126–132.
14. Herter LD, Golendziner E, Flores JA, Moretto M, Di Domenico K, Becker E Jr, et al. Ovarian and uterine findings in pelvic sonography: comparison between prepubertal girls, girls with isolated thelarche, and girls with central precocious puberty. J Ultrasound Med 2002;21:1237–1246.


15. Moon JS, Lee SY, Nam CM, Choi JM, Choe BK, Seo JW, et al. 2007 Korean National Growth Charts: review of developmental process and an outlook. Korean J Pediatr 2008;51:1–25.

16. Greulich WW, Pyle SI. Radiologic atlas of skeletal development of the hand and wrist. 2nd ed. California: Stanford University Press, 1959.
17. Akobeng AK. Understanding diagnostic tests 3: receiver operating characteristic curves. Acta Paediatr 2007;96:644–647.


19. Garel L, Dubois J, Grignon A, Filiatrault D, Van Vliet G. US of the pediatric female pelvis: a clinical perspective. Radiographics 2001;21:1393–1407.


20. Badouraki M, Christoforidis A, Economou I, Dimitriadis AS, Katzos G. Evaluation of pelvic ultrasonography in the diagnosis and differentiation of various forms of sexual precocity in girls. Ultrasound Obstet Gynecol 2008;32:819–827.


21. Fluss R, Faraggi D, Reiser B. Estimation of the Youden Index and its associated cutoff point. Biom J 2005;47:458–472.


22. Haber HP, Mayer EI. Ultrasound evaluation of uterine and ovarian size from birth to puberty. Pediatr Radiol 1994;24:11–13.


23. Griffin IJ, Cole TJ, Duncan KA, Hollman AS, Donaldson MD. Pelvic ultrasound measurements in normal girls. Acta Paediatr 1995;84:536–543.


24. Buzi F, Pilotta A, Dordoni D, Lombardi A, Zaglio S, Adlard P. Pelvic ultrasonography in normal girls and in girls with pubertal precocity. Acta Paediatr 1998;87:1138–1145.


25. Sathasivam A, Rosenberg HK, Shapiro S, Wang H, Rapaport R. Pelvic ultrasonography in the evaluation of central precocious puberty: comparison with leuprolide stimulation test. J Pediatr 2011;159:490–495.


Fig. 1
Ultrasonographic data of the study subjects with central precocious puberty (CPP) and premature thelarche (PT).

Fig. 2
Receiver operator characteristic curves of pelvic ultrasonographic measurements for the diagnosis of central precocious puberty with an area under the curve (95% confidence interval) of 0.588 (0.503-0.673) for uterine length, 0.656 (0.577-0.734) for uterine transverse diameter, 0.660 (0.579-0.741) for fundus, 0.670 (0.593-0.747) for uterine volume, and 0.661 (0.586-0.737) for uterine cross-sectional area (The uterine transverse diameter, fundus, and uterine cross-sectional area are not shown in this graph.).

Fig. 3
Receiver operator characteristic curves of pelvic ultrasonographic measurements for the diagnosis of central precocious puberty in the normal weight group with an area under the curve (95% confidence interval) of 0.663 (0.561-0.765) for uterine length, 0.708 (0.617-0.800) for uterine transverse diameter, 0.727 (0.636-0.817) for fundus, 0.751 (0.666-0.838) for uterine volume, and 0.731 (0.645-0.816) for uterine cross-sectional area (The uterine transverse diameter, fundus, and uterine cross-sectional area are not shown in this graph.).



 PDF Links
PDF Links PubReader
PubReader PubMed
PubMed Download Citation
Download Citation


