Article Contents
| Korean J Pediatr > Volume 59(3); 2016 |
|
Abstract
Purpose
To evaluate the diagnostic value of the Vesikari Scoring System (VSS) as an early predictor of pathogens in children with acute gastroenteritis (AG).
Methods
In this retrospective study, the VSS score, absolute neutrophil count (ANC), and C-reactive protein (CRP) levels were analyzed in 107 hospitalized children with AG, aged 6 months to 17 years. Patients were divided into nonspecific, viral, and bacterial groups according to the pathogens detected using a multiplex polymerase chain reaction (PCR) test.
Results
Patients in the bacterial group had significantly higher CRP values and VSS scores compared to those in the viral group and significantly higher VSS scores compared to those in the nonspecific group (P<0.05). Patients in the viral group had significantly higher VSS scores than those in the nonspecific group (P<0.05). Logistic regression analysis revealed that VSS was the most effective diagnostic tool for predicting the type of pathogen (P<0.05). The area under the receiver operating characteristics curve of VSS was significantly greater than that for ANC and CRP (P<0.05). At a cutoff point of 10 in the VSS, an acceptable diagnostic accuracy could be achieved for distinguishing between bacterial and viral pathogens in AG.
Children are vulnerable to acute gastroenteritis (AG), one of the most common gastro-intestinal diseases. Microbiological investigation is considered in children with underlying chronic conditions or in those who need hospitalization of severe condition, although most AG in children is usually self-limited1). The identification of the pathogen is helpful to determine appropriate therapy and to prevent unnecessary antibiotic treatments, which contribute to the emergence of bacterial resistance and nosocomial transmission2). Appropriate antibiotic therapy can reduce morbidity in some bacterial infection and can be life-saving in invasive AG2).
Physicians often have difficulties in distinguishing between bacterial and viral causes of AG in children. The standard diagnostic measure to identify bacterial pathogens is to perform stool cultures. However, this is time-consuming with a low sensitivity for pathogens. Rapid fecal tests for factors such as occult blood and leukocytes may give contradictory results and are not reliable screening tests for cases of AG3). There is a lack of rapid and accurate diagnostic tools to predict the identity of gut pathogens at an early stage of the disease.
The Vesikari Scoring System (VSS) is the severity scale that was originally developed to evaluate the effectiveness and efficacy of rotavirus vaccines on 20 points4). The parameters and categories of this severity scale are shown in Table 14).
The objective of this study was to evaluate and compare the diagnostic value of the VSS to established infectious markers such as the absolute neutrophil count (ANC) and C-reactive protein (CRP) levels in hospitalized children with AG as an early predictor of pathogen identity.
This retrospective study of patients who were admitted of AG between January 2015 and April 2015 was performed in a single community hospital. AG was defined in patients who have had ≥3 liquid or loose stools with or without vomiting in the preceding 24 hours for less than 7 days5). Patients who had an underlying disease or a noninfectious cause such as acute appendicitis, irritable bowel syndrome, peptic ulcer and gastroesophageal reflux disease were excluded. This study was approved by the Institutional Review Board of KEPCO Medical Center (HIRB-2015-008).
Infectious markers such as the ANC and CRP level were measured in patients' peripheral blood on the first day of admission. One stool sample was collected from each child and divided into 3 portions for the multiplex polymerase chain reaction (PCR) test, stool culture, and measurement of occult blood and leukocytes during the inpatient stay.
For multiplex PCR analyses, the Seeplex Diarrhea ACE Detection Kit (Seegene, Seoul, Korea) was used. The viral pathogens detected by this kit are: rotavirus, norovirus, astrovirus and adenovirus. The bacterial pathogens detected are: Salmonella, Shigella, Vibrio cholerae, Campylobacter, Campylobacter Cx, Clostridium difficile, Clostridium perfringens, Yersinia, Aeromonas hydrophila, Escherichia coli O157, and verotoxin-producing E.coli. Patients were divided into the nonspecific, viral, and bacterial groups according to the pathogen found by the multiplex PCR test. The nonspecific group included patients with no identifiable pathogens on the multiplex PCR test. Patients with both bacterial and viral pathogens were placed in the bacterial group.
Stool cultures were analyzed for Salmonella spp. and Shigella spp. on Salmonella-Shigella agar (Hanil Komed, Sungnam, Korea) and Campylobacter spp. on Campylobacter agars (ASAN Pharmaceutical, Seoul, Korea). Fecal occult blood was evaluated by the NSPlus test (Alfresa, Tokyo, Japan), and fecal leukocytes were examined microscopically.
The VSS scores were determined using the patients' medical records. The following data were collected: patient's age, sex, weight, height, the number and duration of vomiting and diarrhea episodes, the maximum body temperature, severity of dehydration, and treatment modalities. Medical records were reviewed by a pediatrician blinded to the results of the multiplex PCR test.
Data were analyzed using SPSS ver. 17.0 (SPSS Inc., Chicago, IL, USA) and MedCalc, ver. 12.0 (MedCalc, Ostend, Belgium). The ANC, CRP, VSS, fecal occult blood, fecal leukocyte count, and parameters of the VSS in each group were compared using one-way analysis of variance and chi-square tests. The logistic regression analyses and the construction of receiver operating characteristics (ROC) curves with optimal cutoff points in ANC, CRP, and VSS were performed. Statistical significance was assigned to P values of <0.05.
A total of 110 patients were enrolled, but three patients were excluded due to noninfectious causes of their symptoms (acute appendicitis, irritable bowel syndrome, and gastroesophageal reflux disease). A total of 107 patients in the age range 6 months to 17 years (mean±standard deviation: 6.6±5.1) were included with 69 males (64%) and 38 females (36%) (Table 2). The bacterial pathogens were detected in 21 patients (19.6%) and viral pathogens were detected in 63 patients (58.9%) (Table 2). Both bacterial and viral pathogens were detected in 9 patients (8.4%) who were included in the bacterial group. No pathogens were detected in 23 patients (21.5%) who were included in the nonspecific group. We performed stool cultures for Salmonella spp., Shigella spp., and Campylobacter spp. on samples from 44 patients, but no pathogens were identified.
Patients in the bacterial group were found to have significantly higher CRP and VSS values compared to the viral group and significantly higher VSS values compared to the nonspecific group (P<0.05) (Fig. 1). Patients in the viral group were found to have significantly higher VSS than the nonspecific group (P<0.05) (Fig. 1). For fecal occult blood, 23.8% of patients in the bacterial group, 12.7% in the viral group, and 0% in the nonspecific group were positive. The detection rates of fecal leukocytes (at a cutoff of one cell per high-power field) were 19% in the bacterial group, 7.9% in the viral group, and 0% in the nonspecific group. Neither fecal occult blood nor fecal leukocytes showed significant differences among the groups (P>0.05).
The patients in the bacterial group were found to have significantly higher numbers of episodes and longer durations of diarrhea in the parameters of VSS than in the viral and nonspecific groups (P<0.05) (Fig. 2). Patients in the viral group had significantly higher incidences of vomiting than in the bacterial and nonspecific groups (P<0.05) (Fig. 2). However, the duration of vomiting was not significantly different among the groups (P>0.05). There were also no significant differences for maximum body temperature and severity of dehydration (P>0.05).
On the multivariate logistic regression analysis, the VSS was the only statistically significant diagnostic tool for detecting the presence of bacterial pathogens from the viral groups (odds ratio [OR], 2.59; 95% confidence interval [CI], 1.4–4.8) or from the nonspecific groups (OR, 1.3; 95% CI, 1.0–1.5) (P<0.05). The ANC and CRP levels showed no significant predictive significance (P>0.05). The area under the ROC curve (AUC) in the VSS were 0.92 between the bacterial and nonspecific groups, 0.66 between the viral and nonspecific groups, and 0.85 between the bacterial and viral groups (P<0.05) (Fig. 3). The AUC in the ANC and CRP levels amongst the three groups were not statistically significant. Therefore, in the comparisons of ROC curves amongst the VSS, ANC, and CRP values, the VSS was superior to the other tests (Fig. 3). At a cutoff point of 9, the VSS was able to differentiate the bacterial from the nonspecific pathogens in AG with a sensitivity and specificity of 100% and 73.9%, respectively (P<0.05) (Fig. 3). At a cutoff point of 10, the VSS had a sensitivity of 90.5% and a specificity of 60.3% for distinguishing between the bacterial and viral groups (P<0.05) (Fig. 3).
We showed that the VSS could be as useful and reliable as the established infectious markers, ANC and CRP, for predicting the pathogens in pediatric AG. By applying an optimized cutoff point of 10, we would achieve an acceptable overall diagnostic accuracy to distinguish between bacterial and viral pathogens in AG.
CRP measurements have been considered to be useful for detecting the bacterial gastroenteritis, especially in children6). We showed the significant higher means of CRP levels in the bacterial group than in the viral group with the poor diagnostic performance in distinguishing the bacterial and viral pathogens in pediatric AG. An explanation for this might be that CRP is a nonspecific marker for systemic inflammation, and provides limited diagnostic accuracy in detecting specific bacterial infections7). Normal CRP does not exclude the possibility of bacterial gastroenteritis8). There are several studies reporting limited success in using CRP to differentiate bacterial from viral infections6). The data obtained in our analysis demonstrated an advantage of the VSS over CRP in the detection of bacterial AG. ANC measurement is another common laboratory marker used as an acute phase reactant because of the availability of testing. Our findings demonstrated that ANC was found to be less accurate than the VSS and CRP like another studies9).
The fecal occult blood and leukocytes test were demonstrated to have no diagnostic value in identifying the bacterial infections in children with AG in our study. In a meta-analysis assessing the utility of fecal occult blood testing for diagnosing bacterial AG, this test had modestly inferior performance to fecal leukocytes10). Fecal leukocytes had usually been known to be positive in bacterial AG. However, another study reported that the sensitivity of fecal leukocytes varies significantly between inpatients (25%) and outpatients (57%) with similar specificities11). Such poor sensitivity suggests a more limited role for fecal leukocytes in detection of bacterial pathogens in hospitalized patients.
In this study, the VSS was demonstrated to be a practical and accurate diagnostic tool for predicting the pathogens in children with AG. The VSS as a noninvasive test is recommended for children to avoid painful procedures such as venipuncture or invasive endoscopy. The VSS could be useful to standardize assessment and to guide decision making among clinicians with differing levels of training by scoring the symptoms of patients, because the VSS can be calculated using clinical findings by trainees and experienced staff alike. According to the results of this study, we can consider the antibiotic treatment at the cutoff point of 10. We can also use the VSS to evaluate the progress and treatment of AG by recurrent evaluation. However, the weak point of the VSS is that each parameter does not strongly reflect the inflammation suggestive bacterial AG. Therefore, the VSS alone is not likely to change clinical decisions and treatment plans for patients with acute AG. The combinations of infectious makers and VSS are needed to decide how to treat the pediatric AG. Before getting the results from stool culture or stool PCR tests, the VSS could be an additional diagnostic tool with the established infectious markers to draw a treatment plan in pediatric AG.
Our study showed that the viral group was likely to present with vomiting and the bacterial group was likely to present with diarrhea. This result does not mean that clinical features of AG can differentiate a bacterial from a viral etiology8). The bacterial AG, which invades the large bowel, was usually associated with more episodes of diarrhea and longer lasting diarrhea compared with common viral infections. Children with viral AG, which invades the small bowel, presented with more frequent and longer lasting vomiting than children with bacterial AG.
To our knowledge, no other studies have explored the role of the VSS in predicting the pathogens in pediatric AG. We showed that the VSS could accurately differentiate between bacterial and viral pathogens in children with AG after comparing the established infectious markers, ANC and CRP. Our study identified pathogens in 78.5% of patients with AG (21 bacterial and 63 viral). Our identification rate was considerably higher than the 46% reported by other studies based on cultures from a similar age group12). Multiplex PCR used in this study has been shown to be superior to culture for detecting the pathogen in patients with AG13). A limitation of our study was that a small number of patients without healthy controls were enrolled to evaluate the diagnostic performance of the VSS. However, despite of a small number of patients, this study is meaningful because of the first trial of applying the VSS as an early predictor of the pathogen in AG. Another limitation was the nature of the retrospective study design itself. To clarify the diagnostic value of the VSS for predicting the type of pathogens in patients with AG, a prospective multicenter study involving a larger number of patients, including healthy controls, is necessary for a long time.
In conclusion, the VSS could be an additional and helpful diagnostic tool to quickly identify pediatric patients with bacterial AG, accelerating appropriate treatment. We suggest the inclusion of the VSS as a diagnostic aid in clinical decision-making to differentiate subgroups of gut pathogens in children with AG.
Conflicts of interest
Conflict of interest:
No potential conflict of interest relevant to this article was reported.
References
1. Wiegering V, Kaiser J, Tappe D, Weissbrich B, Morbach H, Girschick HJ. Gastroenteritis in childhood: a retrospective study of 650 hospitalized pediatric patients. Int J Infect Dis 2011;15:e401–e407.


3. Mercado EH, Ochoa TJ, Ecker L, Cabello M, Durand D, Barletta F, et al. Fecal leukocytes in children infected with diarrheagenic Escherichia coli. J Clin Microbiol 2011;49:1376–1381.



4. Ruuska T, Vesikari T. Rotavirus disease in Finnish children: use of numerical scores for clinical severity of diarrhoeal episodes. Scand J Infect Dis 1990;22:259–267.


5. Villarruel G, Rubio DM, Lopez F, Cintioni J, Gurevech R, Romero G, et al. Saccharomyces boulardii in acute childhood diarrhoea: a randomized, placebo-controlled study. Acta Paediatr 2007;96:538–541.


6. Marcus N, Mor M, Amir L, Mimouni M, Waisman Y. The quick-read C-reactive protein test for the prediction of bacterial gastroenteritis in the pediatric emergency department. Pediatr Emerg Care 2007;23:634–637.


7. Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med 1999;340:448–454.


8. Guarino A, Ashkenazi S, Gendrel D, Lo Vecchio A, Shamir R, Szajewska H, et al. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition/European Society for Pediatric Infectious Diseases evidence-based guidelines for the management of acute gastroenteritis in children in Europe: update 2014. J Pediatr Gastroenterol Nutr 2014;59:132–152.


9. Chen SM, Ku MS, Lee MY, Tsai JD, Sheu JN. Diagnostic performance of serum interleukin-6 and interleukin-10 levels and clinical predictors in children with rotavirus and norovirus gastroenteritis. Cytokine 2012;59:299–304.


10. Gill CJ, Lau J, Gorbach SL, Hamer DH. Diagnostic accuracy of stool assays for inflammatory bacterial gastroenteritis in developed and resource-poor countries. Clin Infect Dis 2003;37:365–375.


11. Savola KL, Baron EJ, Tompkins LS, Passaro DJ. Fecal leukocyte stain has diagnostic value for outpatients but not inpatients. J Clin Microbiol 2001;39:266–269.



Fig. 1
Comparisons of the ANC (A), CRP levels (B), and VSS scores (C) amongst the nonspecific, viral, and bacterial groups. ANC, absolute neutrophil count; CRP, C-reactive protein; VSS, Vesikari Scoring System.
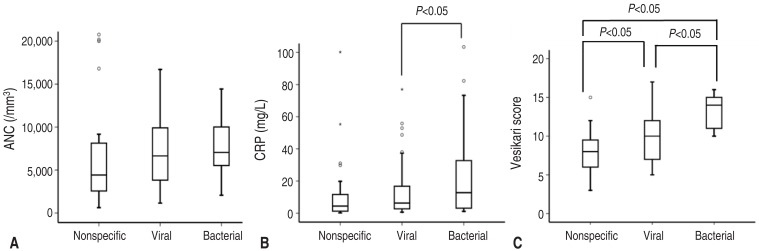
Fig. 2
Comparison of number of diarrhea per day (A), duration of diarrhea (B), number of vomiting per day (C) amongst the nonspecific, viral, bacterial groups.

Fig. 3
Receiver operating characteristics curves of the ANC, CRP levels, and VSS scores between bacterial and nonspecific groups (A), between viral and nonspecific groups (B), and between bacterial and viral groups (C). ANC, absolute neutrophil count; CRP, C-reactive protein; VSS, Vesikari Scoring System; CI, confidence interval.
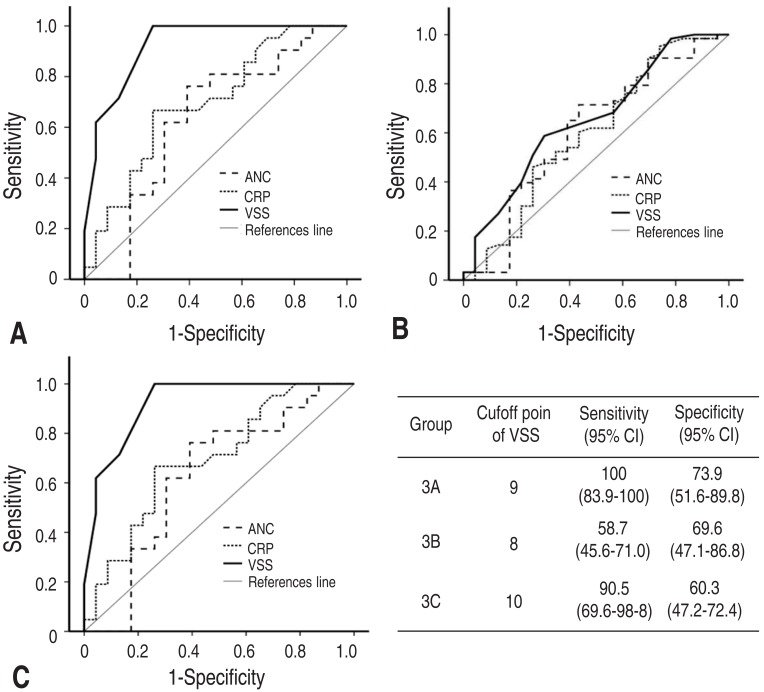
Table 1
Vesikari Scoring System
Adapted from Ruuska T and Vesikari T. Scand J Infect Dis 1990;22:259-674).



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link PubMed
PubMed Download Citation
Download Citation


