Article Contents
| Clin Exp Pediatr > Volume 60(11); 2017 |
|
Abstract
Purpose
To develop and evaluate a simple screening tool to assess hearing loss in newborns. A derived score was compared with the standard clinical practice tool.
Methods
This cohort study was designed to screen the hearing of newborns using transiently evoked otoacoustic emission and auditory brain stem response, and to determine the risk factors associated with hearing loss of newborns in 3 tertiary hospitals in Northern Thailand. Data were prospectively collected from November 1, 2010 to May 31, 2012. To develop the risk score, clinical-risk indicators were measured by Poisson risk regression. The regression coefficients were transformed into item scores dividing each regression-coefficient with the smallest coefficient in the model, rounding the number to its nearest integer, and adding up to a total score.
Results
Five clinical risk factors (Craniofacial anomaly, Ototoxicity, Birth weight, family history [Relative] of congenital sensorineural hearing loss, and Apgar score) were included in our COBRA score. The screening tool detected, by area under the receiver operating characteristic curve, more than 80% of existing hearing loss. The positive-likelihood ratio of hearing loss in patients with scores of 4, 6, and 8 were 25.21 (95% confidence interval [CI], 14.69–43.26), 58.52 (95% CI, 36.26–94.44), and 51.56 (95% CI, 33.74–78.82), respectively. This result was similar to the standard tool (The Joint Committee on Infant Hearing) of 26.72 (95% CI, 20.59–34.66).
Hearing loss was found around 1–3 of 1,000 newborns 1,2,3,4), however in high risk group, the increasing of 10–20 folds was observed1,2). In Thailand, one study performed universal hearing screening in newborns, they found that hearing loss was occurred about 1.7 per 1,000 newborns5). Hearing loss in infants caused delay speech and language development, social and emotional problems, and educational failure 6,7,8,9,10,11). Therefore, early detection of hearing in newborns as soon as possible will lead to early intervention. Particularly in the first 6 months of age, early intervention can help patients and caregivers on the improvement of quality of life, and can reduce the incidence of hearing loss handicap12,13,14,15,16,17,18).
The Joint Committee on Infant Hearing (JCIH)3) recommended universal newborn hearing screening programs to identify all children with significant bilateral hearing loss (30–40 dB or more in the speech range frequency approximately 500–4,000 Hz) up until 3 months of age. Then, treatments or interventions to improve patients' hearing should be started instantly at 6 months of age2,4,19,20,21). Currently, universal hearing loss screening program has not been implemented in Thailand. This might due to limited health care personnel and financial support.
The standard of newborn hearing loss screening is otoacoustic emission (OAE) with auditory brainstem response (ABR)12). However, this approaches need instruments and cannot apply in some setting with limited resources especially in low-and-middle income countries (LMICs), e.g., some primary care settings in Thailand. JCIH (2000) suggested that all newborns age 1 to 28 days should be screened using the following 5 risk factors15); (1) family history of hereditary childhood sensorineural hearing loss, (2) in utero infection: cytomegalovirus, rubella, syphilis, herpes, or toxoplasmosis, (3) craniofacial anomalies, including those with morphological abnormalities of the pinna and ear canal excluding isolated ear pits and tags, (4) admitted to the neonatal intensive care unit (NICU) more than 48 hours or on mechanical ventilation for at least five days, and (5) stigmata or other findings associated with a syndromes associated with congenital hearing loss. In addition, the risk factors of 2007 JCIH12) has changed as the following; NICU care of more than 5 days or any of the following regardless of length of stay: extracorporeal membrane oxygenation, assisted ventilation, exposure to ototoxic medications (gentamycin and tobramycin) or loop diuretics (furosemide), and hyperbilirubinemia that requires exchange transfusion.
However, risk factors screening had never evaluated the worth of early diagnosis and the capability of factors for prevention permanent hearing loss. Furthermore, JCIH risk factor provided the sense of equal point, but in fact it was unequal. Each risk factor has different effect to hearing loss probability. As a results, this study aimed to develop a simple screening score to predict hearing loss, and to serve primary care settings as a screening tool for newborns hearing loss.
The details of study design, study population, and inclusion/exclusion criteria were showed and published elsewhere22). Briefly, data were collated from 3 tertiary hospital located in Northern part of Thailand including Uttaradit Hospital, Budhachinaraj Hospital and Sawanpracharuk Hospital. All newborns who passed the three steps of hearing test were included. The 3-step procedures were transitory evoked OAEs (TEOAEs) (AccuScreen GN Otometrics, PATH medical GmbH, Germering, Germany) followed by automated ABR (AABR) examination (Madsen AccuScreen Otometrics, PATH medical GmbH, Germering, Germany) in case of failed to TEOAE testing. Then children who had deviant AABR results were sent to otolaryngologist for final evaluation with conventional ABR (Sentiero Advanced Otometrics, Germany). ABR more than 25-dB normalized hearing level was considered as hearing loss. Patients were excluded if they did not allowed by parents to participate the study, loss-to-follow up, and die before discharge from the hospital.
Patients' characteristics and all relevant parameters were collected as presented previously22). For example, maternal diseases, family history of congenital sensorineural hearing loss, maternal age, Apgar score at 5 minutes, birth weight, intrauterine infection (maternal infection in uterus with cytomegalovirus, rubella, syphilis, herpes, or toxoplasmosis), craniofacial anomalies, mechanical ventilator use, meningitis, sepsis, ototoxic exposure, delivery route, hyperbilirubinemia. All these factors were used as criteria for infant hearing loss which already mentioned previously12,15).
Standard score was defined as JCIH definition which already stated in introduction part. Derived score was determined using multivariable Poisson risk regression. Only variables which reached statistical significance (P<0.05) were included in the final evaluation. Each variable was weighted by an incident risk ratio coefficient, and transformed to an item score by dividing each regression coefficient with the smallest coefficient in the model and rounding the number to its nearest integer23,24). Cutoff points were determined by statistical significance and the results of the receiver operating characteristic (ROC) curve value. Classical diagnostic values; i.e., specificity, sensitivity, positive predictive value, negative predictive value, likelihood ratio (positive/negative), were computed using a standard method25,26). All P values are 2-tailed and considered statistically significant when the value is less than 0.05. Analyses were performed using STATA ver. 11.0 (StataCorp LP, College Station, TX, USA).
The study was approved by Chiang Mai University Hospital, Uttaradit Hospital, Budhachinaraj Hospital, and Swanpracharuk Hospital Ethical Committee for Clinical Research. All caregivers received all information about the study, and signed an informed consent sheet before participation.
Patients' characteristics were illustrated previously22). Briefly, there was a total of 3,120 infants which 1,534 infants (49%) were male, and 1,586 infants (51%) were female.
Univariable analysis of standard hearing loss screening risk factors found that all factors showed statistical significant (Table 1).
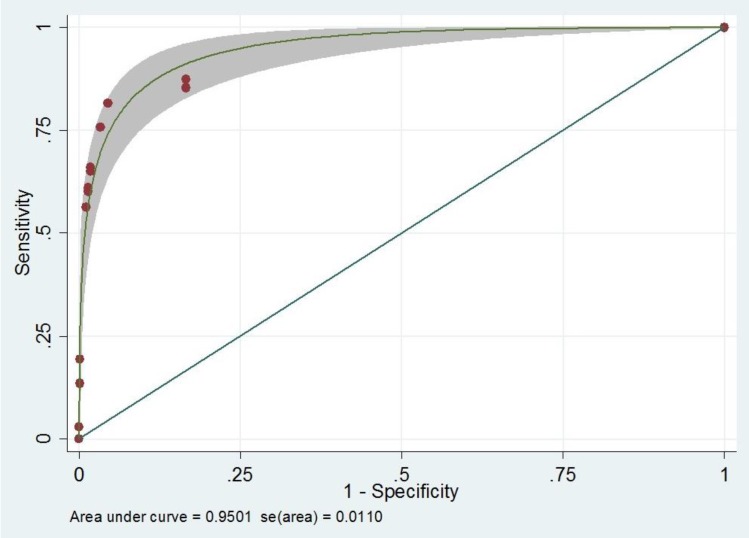
After univariable analysis, multivariable analysis using backward stepwise Poisson regression was performed. There were 5 variables remained in the model to construct a screening scoring system. The screening scoring system for hearing loss is the summation of each point from the following factors; Craniofacial anomaly (yes=4, no= 0), Ototoxicity (yes=9, no=0), Birth weight (low birth weight=1, normal=0), family history (Relative) of congenital sensorineural hearing loss (yes=4, no=0), and Apgar score (abnormal=6.5, normal=0) (Table 2). With this scoring system, the score illustrated a very good performance for hearing loss prediction (area under the ROC [AuROC], 0.95; 95% CI, 0.93–0.97) (Fig. 1). To make an easy way to use and remember, we named this tool as ‘COBRA’ score which derived from initial of each factor.
Performances of diagnostic capability of the screening model compare with standard tool preferred by JCIH were reported in Table 3. Comparing with standard score, COBRA score showed similar results in all diagnostic indices. With this scoring system, the score discriminated patients with hearing loss from those without hearing loos with well validity (AuROC 84%, 88%, 86% for cutoff point at 4, 6, and 8 respectively) (Table 3).
Our study showed that the clinical risk scoring scheme can be used as a screening tool to identify subjects for hearing loss. This score, which we named the “COBRA score”, distinguished the presence or absence of hearing loss in newborns. To our best knowledge, this study is the first clinical score for screening of hearing loss in newborns in Thailand, an Asian countries and one of the LMICs.
In this study, we found 5 clinical risk factors can predict hearing loss in newborns. There were Craniofacial anomaly, Ototoxicity, Birth weight, family history (Relative) of congenital sensorineural hearing loss, and Apgar score. Our finding was similar to those in the previous study in Belgium27) that factors included in the model were family history of hearing loss, low birth weight, low Apgar score. In addition, a study in Thailand19) also showed the similar results to ours that craniofacial anomalies, low birth weight, ototoxic drugs use, are the risk factors of newborns hearing loss. Another one study also used similar four predictors (prematurity, 5-minute Apgar score ≤6, intracranial complication, and craniofacial abnormality) to screen hearing loss in newborns, and showed that the benefit of the screening might motivate parents to follow up with diagnostic ABR testing so that early identification can lead to early intervention28). Although these studies used risk factors for hearing loss screening in newborns, they did not weighted each factor as we did. Lieu et al.28) categorized patients into 3 groups based-on numbers of risk factors. However, since we knew that each risk factor influent the risk of hearing loss as an unequal point, thus counting with the same weight might lead to classification bias.
Considering diagnostic performances of this COBRA score, all diagnostic indices showed a good performance and showed similar results with standard tool of JCIH. Therefore, this COBRA score can be used as newborns screening tool particularly in primary setting where the instruments are scarce.
Strengths of our study should be highlighted. First, we used data from real-world practice, thus this reflect the real situation and can be used in the setting with similar circumstances. Second, we included a number of patients which large enough to capture all related clinical characters. In addition, large sample provided statistical power to diminish random error and bias. Third, this a prospective cohort study, thus data were collected prospectively. This means that information bias, reviewer bias and patient selection bias can be minimized. Forth, the score contains only five clinical parameters which easy to remember as ‘COBRA,’ without additional cost of laboratory testing.
The limitations of this study is that data were collected from only 3 hospitals, thus the findings may not be generalizable to all entries country. However in setting with similar health system, this tool can be applied. Another limitation is external validity. Since this study only develop the score but not validation, thus further validation in other population should be investigated before implementation.
Despite limitation, the simple screening tool (COBRA score) containing five clinical factors for hearing loss in newborns in primary care setting were developed and evaluated. In addition, derived score was also comparable with standard tool currently applied in clinical practice. The medical person in primary care setting can use this screening model for evaluate the hearing of newborn. If the result of screening score was more than 4 points, parents were motivated to bring the children for follow-up with diagnostic ABR testing so that early detection can lead to early intervention.
Notes
Conflicts of interest:
No potential conflict of interest relevant to this article was reported.
References
1. Yoshikawa S, Ikeda K, Kudo T, Kobayashi T. The effects of hypoxia, premature birth, infection, ototoxic drugs, circulatory system and congenital disease on neonatal hearing loss. Auris Nasus Larynx 2004;31:361–368.


2. Wroblewska-Seniuk K, Chojnacka K, Pucher B, Szczapa J, Gadzinowski J, Grzegorowski M. The results of newborn hearing screening by means of transient evoked otoacoustic emissions. Int J Pediatr Otorhinolaryngol 2005;69:1351–1357.


3. Joint Committee on Infant Hearing 1994 Position Statement. American Academy of Pediatrics Joint Committee on Infant Hearing. Pediatrics 1995;95:152–156.

4. Vatovec J, Velickovic Perat M, Smid L, Gros A. Otoacoustic emissions and auditory assessment in infants at risk for early brain damage. Int J Pediatr Otorhinolaryngol 2001;58:139–145.


5. Jariengprasert J. 2003 Universal hearing newborn screening in Ramathibodi hospital. Best Practice in Neonatal Care 2548, :323–365.
6. Prasansuk S. Incidence/prevalence of sensorineural hearing impairment in Thailand and Southeast Asia. Audiology 2000;39:207–211.


7. Thompson DC, McPhillips H, Davis RL, Lieu TL, Homer CJ, Helfand M. Universal newborn hearing screening: summary of evidence. JAMA 2001;286:2000–2010.


8. Mehl AL, Thomson V. The Colorado newborn hearing screening project, 1992-1999: on the threshold of effective population-based universal newborn hearing screening. Pediatrics 2002;109:E7.


9. Valkama AM, Laitakari KT, Tolonen EU, Väyrynen MR, Vainionpää LK, Koivisto ME. Prediction of permanent hearing loss in high-risk preterm infants at term age. Eur J Pediatr 2000;159:459–464.


10. Morton CC, Nance WE. Newborn hearing screening--a silent revolution. N Engl J Med 2006;354:2151–2164.


11. Kochhar A, Hildebrand MS, Smith RJ. Clinical aspects of hereditary hearing loss. Genet Med 2007;9:393–408.


12. American Academy of Pediatrics, Joint Committee on Infant Hearing. Year 2007 position statement: Principles and guidelines for early hearing detection and intervention programs. Pediatrics 2007;120:898–921.


13. US Preventive Services Task Force. Universal screening for hearing loss in newborns: US Preventive Services Task Force recommendation statement. Pediatrics 2008;122:143–148.


14. Nelson HD, Bougatsos C, Nygren P. 2001 US Preventive Services Task Force. Universal newborn hearing screening: systematic review to update the 2001 US Preventive Services Task Force Recommendation. Pediatrics 2008;122:e266–e276.


15. Joint Committee on Infant Hearing. American Academy of Audiology. American Academy of Pediatrics. American Speech-Language-Hearing Association. Directors of Speech and Hearing Programs in State Health and Welfare Agencies. Year 2000 position statement: principles and guidelines for early hearing detection and intervention programs. Joint Committee on Infant Hearing, American Academy of Audiology, American Academy of Pediatrics, American Speech-Language-Hearing Association, and Directors of Speech and Hearing Programs in State Health and Welfare Agencies. Pediatrics 2000;106:798–817.


16. Harrison M, Roush J, Wallace J. Trends in age of identification and intervention in infants with hearing loss. Ear Hear 2003;24:89–95.


17. Geal-Dor M, Levi H, Elidan Y, Arad I. The hearing screening program for newborns with otoacoustic emission for early detection of hearing loss. Harefuah 2002;141:586–590. 668

18. Sun JH, Li J, Huang P, Bu J, Xu ZM, Li J, et al. Early detection of hearing impairment in high-risk infants of NICU. Zhonghua Er Ke Za Zhi 2003;41:357–359.

19. Khaimook W, Chayarpham S, Dissaneevate S. The high-risk neonatal hearing screening program in Songklanagarind Hospital. J Med Assoc Thai 2008;91:1038–1042.

20. Finckh-Krämer U, Gross M, Bartsch M, Kewitz G, Versmold H, Hess M. Hearing screening of high risk newborn infants. HNO 2000;48:215–220.


21. Hess M, Finckh-Krämer U, Bartsch M, Kewitz G, Versmold H, Gross M. Hearing screening in at-risk neonate cohort. Int J Pediatr Otorhinolaryngol 1998;46:81–89.


22. Poonual W, Navacharoen N, Kangsanarak J, Namwongprom S. Risk factors for hearing loss in infants under universal hearing screening program in Northern Thailand. J Multidiscip Healthc 2015;9:1–5.


24. Moons KG, Harrell FE, Steyerberg EW. Should scoring rules be based on odds ratios or regression coefficients? J Clin Epidemiol 2002;55:1054–1055.


25. Janssens AC, Deng Y, Borsboom GJ, Eijkemans MJ, Habbema JD, Steyerberg EW. A new logistic regression approach for the evaluation of diagnostic test results. Med Decis Making 2005;25:168–177.


26. Leisenring W, Pepe MS. Regression modelling of diagnostic likelihood ratios for the evaluation of medical diagnostic tests. Biometrics 1998;54:444–452.





 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link PubMed
PubMed Download Citation
Download Citation


