Article Contents
| Korean J Pediatr > Volume 61(2); 2018 |
|
Abstract
We report the case of a 12-year-old girl who had mild encephalopathy with a reversible splenial lesion (MERS) associated with acutepyelonephritis caused by Escherichia coli. The patient was admitted with a high fever, and she was diagnosed with acute pyelonephritis based on pyuria and the results of urine culture, which detected cefotaxime-sensitive E. coli. Although intravenous cefotaxime and tobramycin were administered, her fever persisted and her C-reactive protein level increased to 307 mg/L. On day 3 of admission, she demonstrated abnormal neuropsychiatric symptoms, such as delirium, ataxia, and word salad. Magnetic resonance imaging (MRI) of the brain performed on day 4 showed marked hyperintensities in the bilateral corpus callosum and deep white matter on diffusion-weighted images, with corresponding diffusion restriction on apparent diffusion coefficient mapping. No abnormalities or pathogens were detected in the cerebrospinal fluid; however, lipopolysaccharides (LPS, endotoxin) were detected in plasma (41.6 pg/mL), associated with acute neurological deterioration. Her clinical condition gradually improved, and no neurological abnormalities were observed on day 6. Follow-up brain MRI performed 2 weeks later showed near-disappearance of the previously noted hyperintense lesions. In this patient, we first proved endotoxemia in a setting of MERS. The release of LPS following antibiotic administration might be related to the development of MERS in this patient. The possibility of MERS should be considered in patients who present with acute pyelonephritis and demonstrate delirious behavior.
Clinically mild encephalitis/encephalopathy with a reversible splenial lesion (MERS), a new clinical-radiological entity, is characterized by magnetic resonance imaging (MRI) findings of transiently reduced diffusion in the splenium of the corpus callosum.1) The lesion may involve the entire corpus callosum and the cerebral white matter in a symmetrical pattern.1) The clinical manifestations consist of relatively mild neurological disorders, including delirium, altered consciousness, and seizures, all of which resolve completely within 1 month.1) Although the pathogenic mechanisms have been not elucidated, infection appears to be associated with MERS. Viruses such as influenza and rotavirus are thought to be common MERS pathogens,1,2) while bacterial infections have been reported in only a few MERS patients.3,4,5) Recently, we experienced MERS in a delirious pediatric patient with acute pyelonephritis caused by Escherichia coli. Although E. coli is a rare cause of MERS,1) this species might be important for understanding the pathogenesis of MERS, because systemic lipopolysaccharides (LPS, endotoxin) can induce brain inflammation and injury.6) We describe the first case of MERS with endotoxemia in a patient with acute pyelonephritis caused by E. coli and discuss its clinical implications, focusing on a possible mechanism of MERS.
A 12-year-old girl was admitted with a high fever for 1 day. She had developed urinary symptoms, including dysuria, a few days earlier. She had an underlying urinary tract anomaly—a duplex kidney with an ectopic ureterocele—and underwent corrective surgery at 4 months of age (Fig. 1). Nevertheless, she suffered from recurrent urinary tract infections since 2 months of age. She also had a history of encephalopathy of unknown cause at 15 months of age and afebrile seizures with rotavirus gastroenteritis at 23 months. No abnormalities on brain MRI and CSF investigations were revealed, but mild electroencephalographic slowing and symptoms such as fever, cluster of seizures, lethargy, and irritability were found at the encephalopathy of 15 months.
The initial laboratory findings were as follows: hemoglobin level, 12.9 g/dL; white blood cell count, 18,340/mm3; platelet count, 267×103/mm3; Na+ level, 136 mEq/L; K+ level, 4.0 mEq/L; and Creactive protein (CRP) level, 81 mg/L. Pyuria was observed. Contrast computed tomography revealed decreased enhancement of the renal parenchyma in the upper pole of the left kidney. She was diagnosed with acute pyelonephritis and treated with intravenous cefotaxime and tobramycin.
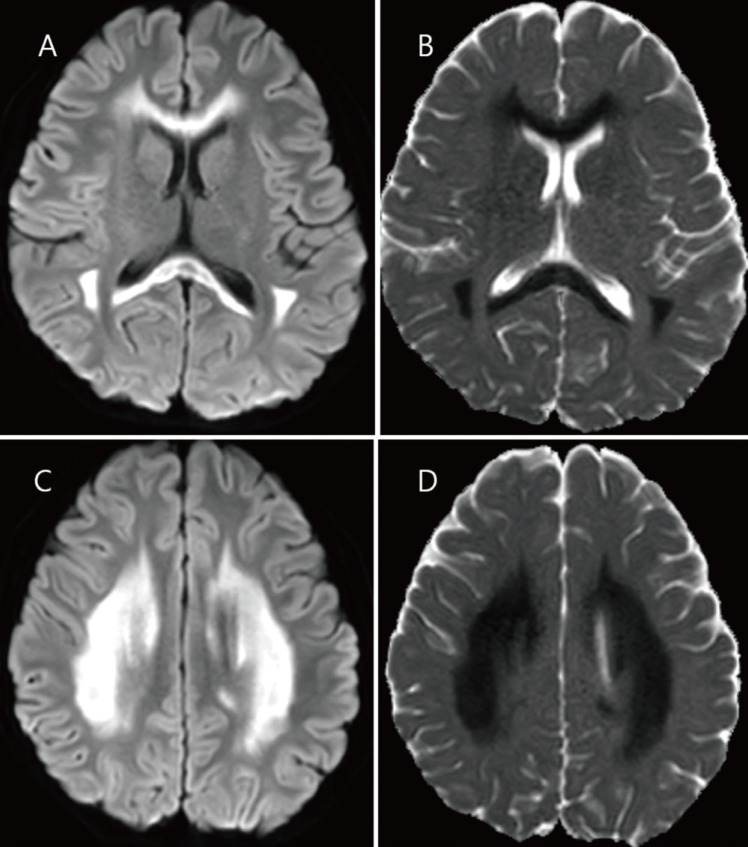
On day 3 of admission, a fever spiking over 39℃ persisted, and the CRP level was increased markedly to 307 mg/L. At this time, delirium, meaningless speech, tremor, and ataxia were observed. She appeared confused and did not respond to questions appropriately. The antibiotics were changed to piperacillin/tazobactam and amikacin. On day 4, the fever began to subside, however, LPS was detected in the plasma (41.6 pg/mL) using enzyme-linked immunosorbent assay (MBS702450, MyBiosource, San Diego, CA, USA). The cerebrospinal fluid (CSF) was normal, with no pleocytosis and a protein level of 18 mg/dL. MRI of the brain performed on day 4 of admission (5 days after her fever began) showed marked symmetric hyperintensity in the corpus callosum and deep white matter bilaterally on diffusion-weighted images, with corresponding diffusion restriction on apparent diffusion coefficient maps (Fig. 2). At that time, third-generation cephalosporin-sensitive E. coli was grown in urine cultures. No viruses (including herpes simplex virus, Ebstein-Barr virus, cytomegalovirus, human herpes virus 6, and enterovirus) or bacteria were isolated from the CSF. Her clinical condition improved gradually. No neurological abnormality was found on day 6 of admission. On follow-up brain MRI on day 18, the hyperintense lesions had mostly disappeared. She was discharged on day 19 with no neurological complications.
An important diagnostic clue of MERS in our case was the obvious delirium, the most common and typical clinical features of MERS.1) However, it might be difficult to recognize neuropsychiatric behavior such as delirium in infants and young children. It has been reported that lethargy, a possible symptoms of MERS, accompanies in about 8% of children with acute pyelonephritis.7) Considering the prevalence of acute pyelonephritis is high in young infants,8) it is possible that MERS associated with acute pyelonephritis is underestimated in pediatric patients. Until now, only a few cases of MERS associated with acute pyelonephritis have been reported in school-age children: 2 caused by Enterococcus faecalis,4) 1 by Klebsiella pneumonia,3) and 2 by E. coli.5)
MERS was observed in a variety of clinical conditions, but in most cases it was associated with viral infection outside the central nervous system (CNS).1) Regarding the mechanisms of MERS, several hypotheses have been suggested such as intramyelinic edema9) and oxidative stress.10) Genetic susceptibility may be involved, particularly in MERS with extensive white matter lesions,11) such as our case. In addition, our patient has had infection-related seizures or encephalopathies; unknown cause of febrile encephalopathy at 18 months, rotavirus-associated seizures at 23 months, and the present episode. Thus, our patient might have a genetic susceptibility to infection-induced acute encephalopathy. However, it is still obscure how non-CNS infection could lead to white matter injury seen in MERS. For this perspective, E. coli might be important for understanding the pathogenesis of MERS.
E. coli accounts for only 5.5% of all causes of MERS,1) however, much evidence shows that systemic LPS exposure can induce brain inflammation and injury, particularly in the white matter.6) Endotoxemia (presence of LPS in the blood) has been considered as one of the causes of brain dysfunction or delirium during sepsis.12) In our patient, endotoxemia was proven at the time of the neuropsychiatric symptoms. LPS is the major component of the outer membrane of gram-negative bacteria, such as E. coli. The administration of antimicrobial agents leads to bacterial cell-wall destruction and the release of LPS.13) In animal models, peripheral injection of this endotoxin initiates an acute inflammatory response, both systemically and centrally.6) LPS can induce the release of proinflammatory cytokines, oxidative radicals, and glutamate via microglial activation.6) Under normal conditions, extremely small amounts of LPS can enter the brain parenchyma.14) However, an animal model of LPS-induced inflammation early in life led to increased blood-brain-barrier permeability later in life.15) Our patient suffered from repeated acute pyelonephritis since infancy. Therefore, LPS itself or the inflammatory cytokines and other substances generated following LPS exposure might have crossed the blood-brain-barrier and mediated inflammation in the brain of our case. The increased CSF levels of inflammatory cytokines are considered to play a critical role in the pathogenesis of MERS, and marked elevation of interleukin-6 has been reported in MERS associated with acute pyelonephritis.4) However, LPS might be coincidentally detected in the present case rather than have a causal relationship with MERS, because endotoxemia is not uncommon in sepsis with pyelonephritis.13) Thus, further studies are needed to verify this issue.
Although E. coli is a very rare cause of MERS, it is an interesting bacterium that could explain the possible mechanism of MERS theoretically. Pediatricians should be aware of the possibility of MERS in a clinical setting of acute pyelonephritis, particularly when unexplained lethargy or delirious behaviors are accompanied.
Acknowledgments
The biospecimens and data used for this study were provided by the Gyeongsang National University Hospital, a member of the Korea Biobank Network.
Notes
Conflicts of interest:
No potential conflict of interest relevant to this article was reported.
References
1. Takanashi J. Two newly proposed infectious encephalitis/encephalopathy syndromes. Brain Dev 2009;31:521–528.


2. Jang YY, Lee KH. Transient splenial lesion of the corpus callosum in a case of benign convulsion associated with rotaviral gastroenteritis. Korean J Pediatr 2010;53:859–862.



3. Okamoto T, Sato Y, Yamazaki T, Hayashi A. Clinically mild encephalitis/encephalopathy with a reversible splenial lesion associated with febrile urinary tract infection. Eur J Pediatr 2014;173:533–536.


4. Kometani H, Kawatani M, Ohta G, Okazaki S, Ogura K, Yasutomi M, et al. Marked elevation of interleukin-6 in mild encephalopathy with a reversible splenial lesion (MERS) associated with acute focal bacterial nephritis caused by Enterococcus faecalis. Brain Dev 2014;36:551–553.


5. Fujiwara Y, Tanaka F, Wakamiya T, Kobori T, Hashiguchi K, Sato M, et al. Four cases of mild encephalitis/encephalopathy with a reversible splenial lesion (MERS) accompanying acute focal bacterial nephritis (AFBN). J Jpn Pediatr Soc 2012;116:1880–1885.
6. Wang X, Rousset CI, Hagberg H, Mallard C. Lipopolysaccharide-induced inflammation and perinatal brain injury. Semin Fetal Neonatal Med 2006;11:343–353.


7. Seidel T, Kuwertz-Bröking E, Kaczmarek S, Kirschstein M, Frosch M, Bulla M, et al. Acute focal bacterial nephritis in 25 children. Pediatr Nephrol 2007;22:1897–1901.


8. Foxman B. Epidemiology of urinary tract infections: incidence, morbidity, and economic costs. Dis Mon 2003;49:53–70.


9. Tada H, Takanashi J, Barkovich AJ, Oba H, Maeda M, Tsukahara H, et al. Clinically mild encephalitis/encephalopathy with a reversible splenial lesion. Neurology 2004;63:1854–1858.


10. Miyata R, Tanuma N, Hayashi M, Imamura T, Takanashi J, Nagata R, et al. Oxidative stress in patients with clinically mild encephalitis/encephalopathy with a reversible splenial lesion (MERS). Brain Dev 2012;34:124–127.


11. Imamura T, Takanashi J, Yasugi J, Terada H, Nishimura A. Sisters with clinically mild encephalopathy with a reversible splenial lesion (MERS)-like features; Familial MERS? J Neurol Sci 2010;290:153–156.


13. Giamarellos-Bourboulis EJ, Perdios J, Gargalianos P, Kosmidis J, Giamarellou H. Antimicrobial-induced endotoxaemia in patients with sepsis in the field of acute pyelonephritis. J Postgrad Med 2003;49:118–122.

Fig. 1
Contrast-enhanced computed tomography image showing decreased enhancement of the renal parenchyma at the upper pole of the left kidney (thick arrow). The renal pelvis in this region of the upper pole appears slightly enlarged without contrast filling (thin arrow). This patient presented with a congenital duplex kidney with an ectopic ureterocele and underwent corrective surgery at 4 months of age.
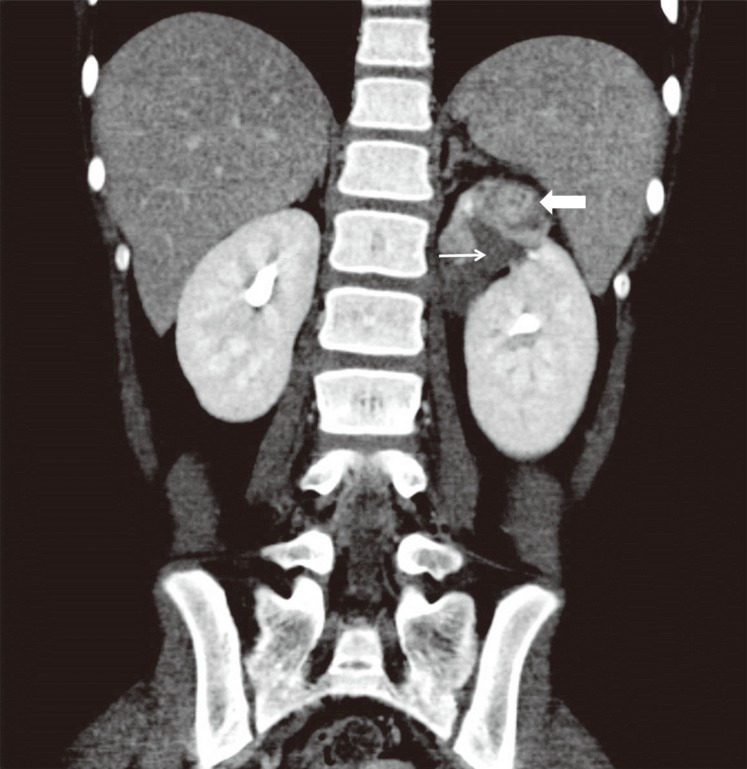
Fig. 2
Brain magnetic resonance images obtained 5 days after fever onset. (A, C) The diffusion-weighted images demonstrate an extensive area of restricted diffusion in the entire corpus callosum and symmetric deep white matter. (B, D) The corresponding diffusion was restricted in the apparent diffusion coefficient map.




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link PubMed
PubMed Download Citation
Download Citation


