Article Contents
| Korean J Pediatr > Volume 61(6); 2018 |
|
Abstract
Purpose
Sacral dimples are a common cutaneous anomaly in infants. Spine ultrasonography (USG) is an effective and safe screening tool for patients with a sacral dimple. The aim of this study was to determine the clinical manifestations in patients with an isolated sacral dimple and to review the management of spinal cord abnormalities identified with USG.
Methods
We reviewed clinical records and collected data on admissions for a sacral dimple from March 2014 through February 2017 that were evaluated with spine USG by a pediatric radiologist. During the same period, patients who were admitted for other complaints, but were found to have a sacral dimple were also included.
Results
This study included 230 infants under 6-months-old (130 males and 100 females; mean age 52.8±42.6 days). Thirty-one infants with a sacral dimple had an echogenic filum terminale, and 57 children had a filar cyst. Twenty-seven patients had a low-lying spinal cord, and only one patient was suspected of having a tethered cord. Follow-up spine USG was performed in 28 patients, which showed normalization or insignificant change.
Cutaneous lesions of the lower back region could be associated with tethered cord syndrome including hairy patches, subcutaneous lipomas, and dimples.1) A simple sacral dimple is less likely to be associated with tethered cord syndrome, but this is difficult to prove. It is not well known whether this is associated with other spinal cord anomalies.
A sacral dimple was found in 1.8%–7.2% of newborn infants;2,3,4) it is a common skin lesion that can easily be found in outpatient clinics or admission during neonatal periods. It has been reported that a dimple can be seen as a typical benign lesion when visible, less than 0.5 cm in size, and has one lesion located in the midline. Large, deep, and distant from the anus, hair, and of changed color may be associated with other diseases.3,4,5) However, further examination is necessary because the visual abnormalities cannot be completely discriminated via visual examination.
Ultrasonography (USG) is a safe and cost-effective screening method and is commonly used in infants with sacral dimples.6,7) It is a noninvasive screening method that does not risk radiation exposure in children, and does not require sedation. In addition, spinal USG performed at a young age is effective because it can acquire relatively accurate imaging compared to postossification. Despite its many advantages, there is a suggestion that USG is not required.8) Currently, limited research exists on the clinical significance of USG in sacral dimple in Korea. This study investigates the clinical features of the sacral dimple in patients with a sacral dimple, and evaluates the prevalence of accompanying diseases and the necessity of USG.
A retrospective review of clinical information and imaging findings (lumbar spinal USG and spine magnetic resonance imaging [MRI]) was performed for the records of 304 children who were diagnosed with a sacral dimple from March 2014 through to February 2017 in Chungnam National University Hospital. A USG was performed for all patients who visited for a sacral dimple. The USG findings of the patients who underwent the first US within 6 months after their birth were analyzed.
Physical examination including height and weight were investigated at the first visit. The birth history including gestational age, birth weight, and delivery method were examined. The clinical features of sacral dimple were determined by examining the hair, skin color, secretion, and distance from the anus to the sacral dimple. Patients with grossly observed anal anomalies or masses, chromosomal anomalies, and multiple deformities were excluded. The flow diagram for enrollment was as drawn in Fig. 1. This study was performed with the approval of the Institutional Review Board of Chungnam National University Hospital (2017-01-022).
All spinal USG were performed by a single pediatric radiologist (SKY) with 5 years of experience using an IU-22 Philips ultrasound system (Philips Healthcare, Bothell, WA, USA) with a linear-array probe (12-5 MHz). A kyphotic curvature was created by placing the patient on a small pillow in a prone position and performing a midline scan over the spinous process. The Normal lumbar spine USG finding was as shown in Fig. 2A. We recorded the level of the tip of the conus medullaris (CM), the pulsation of CM or the nerve roots, the thickness and echogenicity of the filum terminale (FT), the presence of intraspinal mass, and normal variants including filar cysts and ventriculus terminalis.
The echogenicity of FT was compared to adjacent roots of the cauda equina. FT was considered thick when it measured more than 2 mm on transverse and longitudinal US and was considered fibrous or lipomatous nature. We considered it as “prominent FT” when the thickness of the echogenic FT was less than 2 mm. If the tip of the CM was below the L2–3 disc space, this was considered low-lying spinal cord. We defined the isolated low CM as the tip of CM is seen at L2–3 disc space or the L3 vertebral body level without evidence of tethering.9) The findings of additional imaging including follow-up US or MRI were also recorded.
Of the 304 patients who underwent spinal USG, 230 patients (130 boys and 100 girls) who were younger than 6 months were included in the study (Fig. 1). The mean age at first visit was 52.8±42.6 days (range, 1–175 days), the mean height was 56.0±6.1 cm and the mean body weight was 4.9±1.7 kg. In birth history, the mean gestational age was 38.5±1.7 weeks, 29 patients were born as premature neonate. One hundred ninety patients were born at full term, and 1 patient was born at 42 weeks of gestational age. The mean birth weight was 3.1±0.5 kg, and 48% of the patients were delivered via cesarean section. About 65% of the patients visited hospital within 1 month of age (Fig. 3). Thirty-nine patients (17.0%) had hair, and 2 patients (0.9%) had discharge at the dimple region. Twenty patients (8.7%) had skin discoloration and 6 had skin tag (2.6%). The distance from the anal verge to the dimple was 2.14±1.01 cm (Table 1).
During the above-mentioned period, 261 cases of USG were performed with 230 patients, of whom 28 patients underwent follow-up USG (59 cases) and 1 patient underwent a spine MRI. In the first USG, echogenic FT was found in 31 cases (13.5%). Prominent FT (thickness of less than 2 mm, echogenic) was found in 26 cases (mean thickness, 1.4±0.2 mm; range, 1.0–1.9 mm) and 5 cases had a thickness of more than 2 mm (thick FT) (2.4±0.4 mm; range, 2.0–2.9 mm) (Fig. 2B). Filar cyst was identified in 57 cases and their size was 8.6±2.8 mm (range, 3.7–20.0 mm) (Fig. 2C).
The exact CM level could not be confirmed in 6 patients, but their CM levels were considered normal because the CM was visible at the renal hilum level (considered as L1–2 level). When comparing the case of determining the CM level as the renal hilum level and the case of confirming the CM level, 4.6±1.0 months vs. 1.6±1.3 months (P<0.001) indicated that it was difficult to accurately ascertain the CM level after at least 4.6 months. There were 27 cases (11.7%) with low-lying spinal cord (Fig. 2D). There were 26 cases of isolated low CM in which the tip of CM was located in the L2–3 disc space or the middle body of the L3 medullaris. Only one case presented with the CM below the midbody of L3 (Table 2).
Follow-up USG was performed with 28 patients. Eight of 10 patients who were followed up after the echogenic FT were echogenic in follow-up, and 2 patients had normal findings. In 13 patients followed up with filar cyst, 1 was not visible in follow-up, 3 patients were in poor window, and 9 patients (3 times in 2 patients, 20 cases in total) showed follow-up. The thickness of the echogenic FT and filar cyst did not change during the follow-up period.
One patient who had a low lying spinal cord (level L3–4) was suspected of cord tethering on USG and this patient had a small subcutaneous cystic lesion under the coccygeal cartilage without intraspinal extension. The patient was a 4-day-old girl who was delivered by vaginal delivery at a gestational age of 39 weeks 2 days and at a birth weight of 2.12 kg. She was admitted to neonatal intensive care unit for intrauterine growth retardation, and USG was performed for sacral dimple, which was found incidentally during hospitalization. A sacral dimple was located in the midline, 3 mm from the anal verge. As described above, abnormal findings were detected on spinal USG and spine MRI was performed for further evaluation. The patient was referred to a neurosurgeon; additional testing was recommended including contrast enhancement MRI and surgery was considered. However, the caregiver refused further evaluation and was the patient was discharged on his own accord.
The sacral dimple is one of the most common skin lesions, but it is a simple skin lesion in most cases and does not affect neurologic dysfunction. In our study, half of the patients showed normal USG finding without anomaly. The other patients had filar cyst (24.8 %), echogenic FT (13.5%), and low-lying spinal cord (11.7%). Considering that filar cysts are also classified as normal findings in other literature,10) 74.8% of patients had normal USG findings among our patients. Although patients had abnormal findings for USG, physical examination and observational findings were nonspecific except in 1 patient.
One of the major reasons for performing USG is early detection of the possibility of tethered cord syndrome. A tethered cord syndrome is caused by a stretch-induced dysfunction of the caudal spinal cord and conus, that often associated with spinal dysraphism.11) In children, tethered cord syndrome typically present with progressive motor and sensory dysfunction, which may include gait abnormalities and urologic symptoms.12) Although there are some asymptomatic patients with anatomic cord tethering, the patients with developed symptoms does not naturally improve without surgical untethering.13) And early intervention after symptom development is important for recovery of neurological functions.13,14,15) Early diagnosis is also necessary for prevent neurological damage and adequate surgical correction.16)
In a review article, the incidence of abnormalities in spinal USG in children without dimple was 4.8%, which was not significantly different from 3.8% in children with a dimple.8) This leads to questions about whether ultrasound should be performed in patients with simple sacral dimples.17,18,19,20,21) Although only one patient among 230 needed a surgical procedure in our study, considering neurologic problems caused by tethered cord and importance of early diagnosis, USG is worthy for the screening of sacral dimples in infants. In one study of comparing USG and MRI, USG is valuable diagnostic tool for congenital anomalies of the lower spine in infants.22) It is meaningful to perform an USG in Korea, considering that the cost of medical services is not expensive compared to the United States or Europe, and hospitals are readily accessible.23) Furthermore, Ohashi et al.24) reported a case of mucopolysaccharidosis type IV, which was diagnosed during sacral dimple evaluation at birth. Prompt evaluation could lead to the identification of other treatable diseases in patients.
Cord tethering can occur by FT lipoma, which is the most common cause of thick FT. Therefore, detection of abnormal FT thickening is important.25) In most of the literature, a thickness of FT of more than 2 mm was considered thick FT. Shin et al.25) compared lumbosacral USG and MRI findings and suggested 1.1 mm as the optimal cutoff value for filar lipoma screening on USG. We observed prominent FT in cases of echogenic rather than nerve root, and thick FT in cases in which the thickness was more than 2 mm. The incidence was 11.3% and 2.2%, respectively.
Irani et al.10) reported that the frequency of filar cysts was 11.8 %, and the short-term results were not significantly different from those of the normal controls. Although the origin and existence of filar cysts is not well studied, neonatal filar cysts found in isolation on lumbar USG can be considered as normal variants. In our study, the incidence of filar cysts in patients with a sacral dimple was 24.8%. Our findings showed higher prevalence than Irani et al.10); their study included more than 600 patients and infants older than 12 months were included. There were differences in the number and ages of patients, and no specific symptoms were observed in our patients.
Our study only included patients less than 6 months old to ensure the accuracy of ultrasound images. However, there were patients who visited with a sacral dimple even after 6 months of age. Including these patients makes the mean age of the patients who visited the hospital with a sacral dimple 2.7±4.6 months (range, 0–62.8 months); 89.5% of the patients visited the hospital 6 months after birth, and 75.1% of patients visited the hospital 3 months after birth. Most of the patients had a sacral dimple during the physical examination for neonatal examination, national infant screening, and vaccination. In our study, 129 patients (56.1%) were normal and the other 100 patients had no neurological abnormalities associated with sonographic findings.9,10,26,27) In some cases, the patients did not revisit the clinic even though follow-up tests were planned. This might be because the parents of the patients thought that their baby had no neurological abnormalities. No significant changes were found in the 28 children who underwent a follow-up.
The limitations of this study included that it was a retrospective, single-institutional study. The case could be made that the medical records were insufficient because of the recruitment of retrospective subjects. Future prospective studies should identify long-term clinical outcomes. Infants after 3 months had fewer than other age groups, because progressed ossification made ultrasound imaging difficult. Overcoming regional biases requires investigating the clinical manifestations of the sacral dimple in Korea by studying more patients in multiple institutions. In conclusion, most of the patients in this study had a good clinical course; we found 1 case of abnormality that could lead to neurologic abnormalities in this study. Therefore, USG screening tests might be useful for children with sacral dimples.
Acknowledgments
This work was supported by a research fund from the Chungnam National University (2014-0656-01). This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Science, ICT & Future Planning (grant number: 2015R1C1A1A01052351). This work was presented at the Korean Pediatric Society Congress in 2016.
Notes
Conflicts of interest:
No potential conflict of interest relevant to this article was reported.
References
1. Chern JJ, Kirkman JL, Shannon CN, Tubbs RS, Stone JD, Royal SA, et al. Use of lumbar ultrasonography to detect occult spinal dysraphism clinical article. J Neurosurg Pediatr 2012;9:274–279.


2. Henriques JG, Pianetti G, Henriques KS, Costa P, Gusmão S. Minor skin lesions as markers of occult spinal dysraphisms: prospective study. Surg Neurol 2005;63(Suppl 1): S8–S12.


3. Kriss VM, Desai NS. Occult spinal dysraphism in neonates: assessment of high-risk cutaneous stigmata on sonography. AJR Am J Roentgenol 1998;171:1687–1692.


4. Sarikaya Solak S, Kivanc Altunay I, Tukenmez Demirci DG, Can B. Prevalence of congenital cutaneous anomalies in 1000 newborns and a review of the literature. Am J Perinatol 2016;33:79–83.

5. Kucera JN, Coley I, O'Hara S, Kosnik EJ, Coley BD. The simple sacral dimple: diagnostic yield of ultrasound in neonates. Pediatr Radiol 2015;45:211–216.


6. Medina LS, Crone K, Kuntz KM. Newborns with suspected occult spinal dysraphism: a cost-effectiveness analysis of diagnostic strategies. Pediatrics 2001;108:E101.


7. Ben-Sira L, Ponger P, Miller E, Beni-Adani L, Constantini S. Low-risk lumbar skin stigmata in infants: the role of ultrasound screening. J Pediatr 2009;155:864–869.


8. Albert GW. Spine ultrasounds should not be routinely performed for patients with simple sacral dimples. Acta Paediatr 2016;105:890–894.


9. Thakur NH, Lowe LH. Borderline low conus medullaris on infant lumbar sonography: what is the clinical outcome and the role of neuroimaging follow-up? Pediatr Radiol 2011;41:483–487.


10. Irani N, Goud AR, Lowe LH. Isolated filar cyst on lumbar spine sonography in infants: a case-control study. Pediatr Radiol 2006;36:1283–1288.


11. Michelson DJ, Ashwal S. Tethered cord syndrome in childhood: diagnostic features and relationship to congenital anomalies. Neurol Res 2004;26:745–753.


12. Hertzler DA 2nd, DePowell JJ, Stevenson CB, Mangano FT. Tethered cord syndrome: a review of the literature from embryology to adult presentation. Neurosurg Focus 2010;29:E1.

13. Lew SM, Kothbauer KF. Tethered cord syndrome: an updated review. Pediatr Neurosurg 2007;43:236–248.


14. Cornette L, Verpoorten C, Lagae L, Van Calenbergh F, Plets C, Vereecken R, et al. Tethered cord syndrome in occult spinal dysraphism: timing and outcome of surgical release. Neurology 1998;50:1761–1765.


15. van der Meulen WD, Hoving EW, Staal-Schreinemacher A, Begeer JH. Analysis of different treatment modalities of tethered cord syndrome. Childs Nerv Syst 2002;18:513–517.


16. Lode HM, Deeg KH, Krauss J. Spinal sonography in infants with cutaneous birth markers in the lumbo-sacral region: an important sign of occult spinal dysrhaphism and tethered cord. Ultraschall Med 2008;29(Suppl 5): 281–288.

17. Sneineh AK, Gabos PG, Keller MS, Bowen JR. Ultrasonography of the spine in neonates and young infants with a sacral skin dimple. J Pediatr Orthop 2002;22:761–762.


18. Block SL. The enigmatic sacro-coccygeal dimple: to ignore or explore? Pediatr Ann 2014;43:95–100.


19. Robinson AJ, Russell S, Rimmer S. The value of ultrasonic examination of the lumbar spine in infants with specific reference to cutaneous markers of occult spinal dysraphism. Clin Radiol 2005;60:72–77.


20. Wilson P, Hayes E, Barber A, Lohr J. Screening for spinal dysraphisms in newborns with sacral dimples. Clin Pediatr (Phila) 2016;55:1064–1070.


21. Gibson PJ, Britton J, Hall DM, Hill CR. Lumbosacral skin markers and identification of occult spinal dysraphism in neonates. Acta Paediatr 1995;84:208–209.


22. Rohrschneider WK, Forsting M, Darge K, Tröger J. Diagnostic value of spinal US: comparative study with MR imaging in pediatric patients. Radiology 1996;200:383–388.


23. Zanello M, Zerah M, Di Rocco F. Sacral dimple: what form of management is best? Arch Pediatr 2015;22:1298–1301.


24. Ohashi A, Montaño AM, Colón JE, Oguma T, Luisiri A, Tomatsu S. Sacral dimple: incidental findings from newborn evaluation. Mucopolysaccharidosis IVA disease. Acta Paediatr 2009;98:768–769. 910–912.


25. Shin HJ, Kim MJ, Lee HS, Kim HG, Lee MJ. Optimal filum terminale thickness cutoff value on sonography for lipoma screening in young children. J Ultrasound Med 2015;34:1943–1949.


Fig. 1
Flow diagram showing the enrollment of patients with a sacral dimple. CNS, central nervous system.
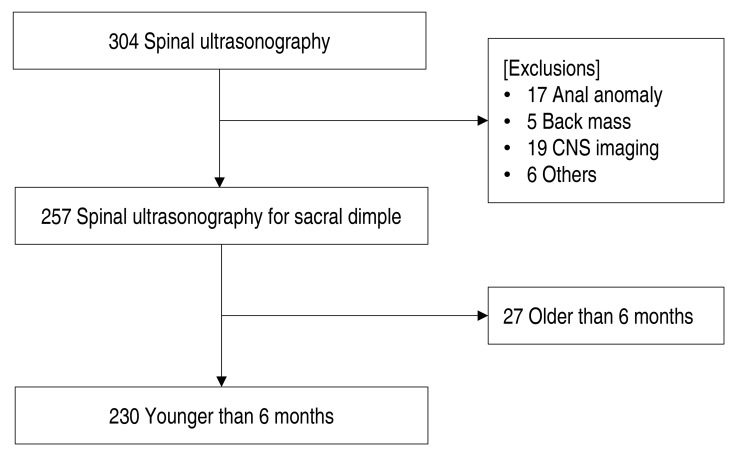
Fig. 2
Ultrasonography (USG) findings in patients. (A) Normal lumbar spine USG in a 4-day-old boy. Longitudinal USG shows normal anatomy of the spinal canal and its contents. (B) Low-lying spinal cord in a 4-day-old girl. (B1) Longitudinal USG shows the tip of the conus medullaris terminating at L3–4 disc space. (B2) Sagittal T2-weighted magnetic resonance imaging confirms the level of the conus medullaris at the L3–4 disc space. (C) Echogenic filum terminale. (C1) Prominent filum terminale in a 6-month-old boy. Longitudinal USG shows hyperechoic filum terminale with normal thickness (about 1.3 mm). (C2) Thick filum terminale in a 5-month-old boy. Longitudinal USG shows hyperechoic filum terminale with 2.2-mm thickness. (D) Filar cyst (arrow in D1 and D2) in a 1-month-old boy. Longitudinal (D1) and transverse (D2) USG shows well-defined, thin walled, fusiform cyst below the tip of the conus medullaris.







 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link PubMed
PubMed Download Citation
Download Citation


