Article Contents
| Korean J Pediatr > Volume 59(Suppl 1); 2016 |
|
Abstract
Infectious mononucleosis is Epstein-Barr virus (EBV) inducing a self-limiting clinical syndrome characterized by fever, sore throat, hepatosplenomegaly, and generalized lymphadenopathy. Gastrointestinal symptoms of EBV infection are nonspecific and occur rarely. EBV inducing acute gastrointestinal pathology is poorly recognized without suspicion. Careful consideration is needed to diagnose gastric involvement of EBV infection including gastric lymphoma, gastric cancer, and gastritis. A few recent cases of gastritis associated with EBV infection have been reported in adolescents and adults. However, there is no report of EBV-associated gastritis in early childhood. We experienced a rare case of 4-year-old girl with EBV gastritis confirmed by in situ hybridization.
The Epstein-Barr virus (EBV), which belongs to the γ-herpesviridae family, causes a persistent and lifelong latent infection in patients worldwide. Infectious mononucleosis, the well-known related clinical syndrome, is triggered by the EBV1). Its main presenting symptoms are fatigue, malaise, sore throat, and generalized lymphadenopathy2). Hepatic involvement may occur as symptoms of EBV infection, including hepatomegaly, jaundice, and abnormal liver function test3). Although gastric involvement is extremely rare, gastritis, lymphoma, and gastric cancer can develop. Concomitant acute gastritis rarely occurs, and only a few cases have been reported in adults and adolescents. There have been no case reports to date in children. We report a case of EBV gastritis documented in a 4-year-old girl who presented with fever, cervical lymphadenopathy, and abdominal pain.
A 4-year-old girl was hospitalized via the Emergency Department with a 6-day history of fever, headache, and a 3-day history of upper and periumbilical abdominal pain. There was no vomiting and diarrhea, but the abdominal pain was aggravated with the complaint of anorexia. She had a history of febrile convulsion and tic disorder. No notes about supplementary food or special doses of medicines were found.
Physical examination showed a temperature of 38.5℃, blood pressure of 114/63 mmHg, heart rate of 96 beats/min, and respiratory rate of 24 breaths/min. Pharyngeal injection and cervical lymphadenopathy were observed. The abdomen was flat and soft. Upper quadrant and periumbilical tenderness were noted with normal bowel sounds. The liver edge was palpable 5 cm below the right low costal margin. The spleen was enlarged. There was no skin rash.
The cell blood count examination revealed the following; white blood cell count 11,120/µL (segment, 6.0%; lymphocyte, 40.0%; atypical lymphocyte, 37.0%), hemoglobin level 11.4 g/dL, hematocrit 33.1%, and platelet count 254,000/µL. Results of liver function tests were abnormal, including aspartate transaminase (105 IU/L), alanine transaminase (166 IU/L), alkaline phosphatase (363 IU/L), γ-glutamyl transpeptidase (229 IU/L), and albumin (3.4 g/dL). Other results were as follows: serum lactate dehydrogenase 799 IU/L and total bilirubin 0.6 mg/dL. Serologic tests were negative for hepatitis B viral surface antigen as well as antibodies against hepatitis A virus, hepatitis C virus, and cytomegalovirus. Blood culture failed to grow any bacteria. Monospot testing for heterophil antibody was negative. Serum IgM antibody for EBV capsid antigen and IgG antibody for EBV nuclear antigen were negative. Polymerase chain reaction performed with peripheral whole blood was positive for EBV DNA with a viral load of 8,180 copies/mL.
Abdominal ultrasonography showed hepatosplenomegaly, multiple enlarged lymph nodes along the greater curvature of the stomach, and enlarged periportal and ileocolic lymph nodes. Diffuse thickening of the gastric wall and prominent enhancement of the mucosal layer and multiple enlarged lymph nodes along to the greater curvature of stomach were detected on a computed tomography scan, suggesting lymphoproliferative disease and infectious disease (Fig. 1). Due to li2016-12-16mitations of the radiographic findings for differentiating the lymphoproliferative disease and infectious disease, esophagogastroduodenoscopy (EGD) with biopsy was performed, which revealed edematous mucosa in the antrum and body of the stomach. The esophageal and duodenal mucosa findings were nonspecific. Since endoscopic findings were not suggested for gastric lymphoproliferative disease, we easily thought the possibility of EBV gastritis. Histological findings of hematoxylin and eosin staining revealed expansion of the lamina propria and a proliferation of atypical lymph nodes in the mucosa (Fig. 2A). No eosinophils were noted on biopsies. Immunohistochemical staining showed the expression of each T-cell marker (CD3 and CD45RO) and B-cell marker (CD20 and CD79a) (Fig. 2B, C). An EBV-encoded small RNA in situ hybridization test showed positive lymphocytes in the lamina propria (Fig. 2D). A campylobacter-like organism test was negative for Helicobacter pylori. The abdominal pain and sore throat alongside with cervical lymphadenopathy improved with histamine 2 (H2) receptor antagonist and supportive care. The patient was discharged on the 10th day of hospitalization. Two months after discharge, the edematous gastric antral and body mucosa were normal on a follow-up EGD. The biopsy showed no atypical lymph node proliferation of the gastric mucosa and an in situ hybridization test was negative.
We experienced a rare case of EBV gastritis that developed in a healthy child as a manifestation of infectious mononucleosis secondary to EBV infection. The diagnosis was established by proving EBV in gastric biopsies with in situ hybridization, and the patient recovered spontaneously due to the infection's self-limiting nature.
As noted previously, EBV gastritis is rarely reported. We found only 6 case reports of EBV gastritis in the literature (Table 1)1,4,5,6,7,8). Here we presented a case of EBV gastritis in a previously well 4-year-old girl. To our knowledge, this is the first case report of EBV gastritis in a young child.
EBV infection-related diseases include infectious mononucleosis, multiple organ disease, complications caused by acute infection, lymphoproliferative disease, and certain types of cancer9). Infectious mononucleosis is an acute disease caused by EBV infection. Its features are atypical lymphocytosis on peripheral blood, fever, and generalized lymphadenopathy10). As mentioned above, our patient had typical clinical symptoms and signs of infectious mononucleosis but other previous reports were not. There were no typical symptoms and signs of infectious mononucleosis in previous reports but their patients all had atypical lymphocytosis on peripheral blood. Related gastrointestinal cancers include gastric non-Hodgkin's lymphoma, gastric T-cell lymphoma, gastric adenocarcinoma, and esophageal cancer11,12,13). Neurologic or blood disorders rarely occur14,15).
Making the clinical diagnosis of EBV infection is usually simple. It can be done by serological tests for EBV and extracting heterophil antibody. Serum specific antibodies for EBV are viral capsid antigen IgM/IgG, EBV early antigen IgG/IgM, and Epstein-Barr nuclear antigen IgG. Polymerase chain reaction for EBV DNA is also available. Our patient had fever, sore throat, and cervical lymphadenopathy. Although the specific serum antibodies were all negative, the diagnosis of infectious mononucleosis was confirmed by the presence of EBV load in assays of DNA extracted from the peripheral blood.
EBV infection is simply diagnosed with serological testing. However, EBV gastritis can occur without positive serological evidence. As such, it is possible to overlook EBV even in patients with gastrointestinal symptoms. Our case along with the previous cases shows that EBV gastritis selectively invades the stomach without involving lesions of the esophagus and duodenum1,6).
EBV gastritis might be confused with various types of lymphoma radiographically, histologically, and endoscopically. Kitayama et al.6) presented that it could be mis-diagnosed as diffuse B-cell lymphoma at the first assessment. Moreover, EBV gastritis can involve acute or chronic in nature, and it is difficult to distinguish it from other types of gastritis such as Helicobacter gastritis and lymphocytic gastritis2,8). Various reports of endoscopic findings stated that EBV gastritis was accompanied by a range of conditions from mucosal hypertrophy with partial edema to ulcerative lesions with irregular margins1).
Since endoscopic appearances of EBV gastritis were various as shown in other cases, performing biopsies with in situ hybridization could be helpful for confirming the diagnosis. In our case, EGD was performed to evaluate the diffuse thickening of the gastric wall visible on imaging studies. EBV gastritis was diagnosed by biopsies with in situ hybridization based on our suspicion judging by the patient's clinical manifestation. And our patient was recovered with using H2 receptor antagonist and supportive care, which was consistent with previous reports1,5,6,7,8).
In summary, we reported a rare case of EBV gastritis in a 4-year-girl presenting with upper and periumbilical abdominal pain as well as fever, sore throat, and cervical lymphadenopathy. This is the first case of EBV gastritis in an early childhood. EBV-associated gastric involvement was carefully investigated in the case of upper digestive symptoms in the clinical situation of highly suspicious EBV infection.
Serological testing for EBV infection and biopsies with in situ hybridization by EGD are useful tools to clarify EBV-associated gastric involvement.
Notes
Conflict of interest:
No potential conflict of interest relevant to this article was reported.
References
1. Zhang Y, Molot R. Severe gastritis secondary to Epstein-Barr viral infection. Unusual presentation of infectious mononucleosis and associated diffuse lymphoid hyperplasia in gastric mucosa. Arch Pathol Lab Med 2003;127:478–480.

2. Rea TD, Russo JE, Katon W, Ashley RL, Buchwald DS. Prospective study of the natural history of infectious mononucleosis caused by Epstein-Barr virus. J Am Board Fam Pract 2001;14:234–242.

3. Markin RS. Manifestations of Epstein-Barr virus-associated disorders in liver. Liver 1994;14:1–13.


4. Owens SR, Walls A, Krasinskas AM, Rund CR. Epstein-Barr virus gastritis: rare or rarely sampled? A case report. Int J Surg Pathol 2011;19:196–198.


5. Chen ZM, Shah R, Zuckerman GR, Wang HL. Epstein-Barr virus gastritis: an underrecognized form of severe gastritis simulating gastric lymphoma. Am J Surg Pathol 2007;31:1446–1451.


6. Kitayama Y, Honda S, Sugimura H. Epstein-Barr virus-related gastric pseudolymphoma in infectious mononucleosis. Gastrointest Endosc 2000;52:290–291.


7. Sujino T, Ebinuma H, Hosoe N, Okamoto S, Imaeda H, Hayashi Y, et al. Epstein-barr virus-associated gastritis: a case report. Dig Dis Sci 2013;58:883–886.


8. Hisamatsu A, Nagai T, Okawara H, Nakashima H, Tasaki T, Nakagawa Y, et al. Gastritis associated with Epstein-Barr virus infection. Intern Med 2010;49:2101–2105.


9. Yanai H, Takada K, Shimizu N, Mizugaki Y, Tada M, Okita K. Epstein-Barr virus infection in non-carcinomatous gastric epithelium. J Pathol 1997;183:293–298.


11. Ueo T, Kashima K, Daa T, Kondo Y, Yokoyama S. Coexistence of Epstein-Barr virus-associated gastric carcinoma with malignant lymphoma: report of two cases. Virchows Arch 2006;449:215–219.


12. Thorley-Lawson DA, Gross A. Persistence of the Epstein-Barr virus and the origins of associated lymphomas. N Engl J Med 2004;350:1328–1337.


13. Mori M, Watanabe M, Tanaka S, Mimori K, Kuwano H, Sugimachi K. Epstein-Barr virus-associated carcinomas of the esophagus and stomach. Arch Pathol Lab Med 1994;118:998–1001.

Fig. 1
Diffuse wall thickening of the stomach (*) detected by computed topographic scan (A), and diffuse edematous gastric mucosa was noted in the body and antrum (B).

Fig. 2
Microscopic findings. (A) There is diffuse atypical lymphocytes proliferation in the lamina propria (H&E, ×100). (B, C) These cells are positive for CD20 (B) and CD3 (C) (immunohistochemistry, ×200). (D) It shows also positive for Epstein-Barr virus-encoded small RNA staining (in situ hybridization method, ×200)
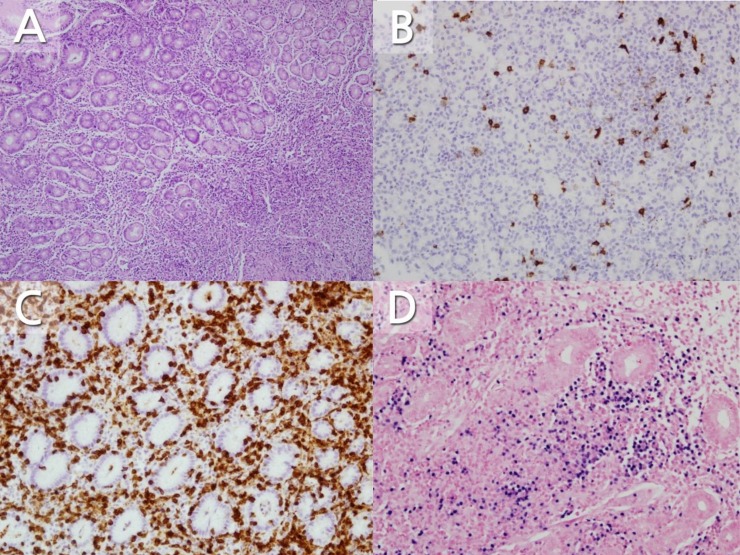
Table 1
Clinical features of Epstein-Barr virus gastritis
| Study (yr) | Age (yr)/sex | Symptoms | Endoscopic findings | Histology | Helicobacter pylori |
|---|---|---|---|---|---|
| Kitayama et al.6) (2000) | 40/M | Fever, diarrhea | Multiple small irregular ulcers |
Diffuse atypical lymphocytic infiltration CD79a+/CD20+ In situ hybridization+ |
Negative |
| Zhang et al.1) (2003) | 58/unknown | Abdominal pain, anorexia, vomiting, diarrhea | Thickened, edematous and inflamed rugal folds without ulcer |
Prominent lymphoid infiltration CD20+/CD3– In situ hybridization+ |
Negative |
| Chen et al.5) (2007) | 59/M | Epigastric pain, fever, nausea | Diffusely granular and erythematous, mucosa and numerous ulcers |
Diffuse atypical lymphocytic infiltration CD3+/CD20+ In situ hybridization+ |
Negative |
| Hisamatsu et al.8) (2010) | 17/F | Epigastric pain, fever, sore throat | Granular mucosa and erosion |
Dense diffuse lymphoid infiltration CD79a+/CD45RO+ In situ hybridization+ |
Negative |
| Owens et al.4) (2011) | 18/F | Epigastric pain, fever, vomiting | Nodular mucosa and a solitary ulcer |
Atypical immunoblast like cells CD3+/CD20+ In situ hybridization+ |
Positive |
| Sujino et al.7) (2013) | 37/M | Abdominal symptom, fever, sore throat | Gastric ulcers with irregular margin |
Atypical infiltration inflammatory cells CD 9a+/CD20+/CD3+ In situ hybridization+ |
Negative |
| Present case (2014) | 4/F | Periumbilical pain, fever, headache | Edematous rugal folds with mucosal swelling |
Atypical lymphoid cell infiltration CD3+/CD45RO+/CD20+/CD79a+ In situ hybridization+ |
Negative |



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link PubMed
PubMed Download Citation
Download Citation


