Article Contents
| Clin Exp Pediatr > Volume 59(Suppl 1); 2016 |
|
Abstract
Neuroblastomas are sometimes associated with abnormal constitutional karyotypes, but the XYY karyotype has been rarely described in neuroblastomas. Here, we report a case of an esthesioneuroblastoma in a boy with a 47, XYY karyotype. A 6-year-old boy was admitted to our hospital because of nasal obstruction and palpable cervical lymph node, which he first noticed several days previously. A polypoid mass in the right nasal cavity was detected through sinuscopy. Biopsy of the right nasal polyp was performed. Based on the result, the patient was diagnosed with a high-grade esthesioneuroblastoma. Nuclear imaging revealed increased uptake in both the right posterior nasal cavity and the right cervical IB-II space, suggesting metastatic lymph nodes. Cytogenetic analysis revealed a 47, XYY karyotype. Twelve courses of concurrent chemotherapy were administered. Three years after the completion of chemotherapy, the patient had had no disease recurrence. He manifested behavioral violence and temper tantrums, so we started methylphenidate for correction of the behavior.
Some constitutional chromosomal abnormalities are known to have an increased risk of malignancies1). For examples, the patients with Kleinfelter's syndrome (47, XXY) have an increased risk of developing breast cancer or mediastinal germ cell tumors2,3). The children with Down syndrome (47, XY, +21) have a 10- to 20-fold increased risk of developing acute lymphoblastic leukemia and acute myeloid leukemia4,5). XYY karyotype has an incidence of one in 1,000 male newborns, which may result from a nondisjunction in paternal meiosis II or postzygotic mitotic nondisjunction6). The XYY karyotype is known as an incidental finding unrelated to the malignant process, so the association of XYY karyotype and malignancy is rarely reported in literatures7,8). Whether XYY karyotype is a random event or the extra Y chromosome predisposes to malignancies has been controversial.
In solid tumor, dysembryoplastic neuroepithelial tumor (DNT) associated with the XYY karyotype was reported9). Neuroblastoma, which is the most common extracranial solid tumor in children, is sometimes associated with abnormal constitutional karyotype, but neuroblastoma associated with the XYY karyotype was described only one case10,11). Another case of neuroblastoma with XYY karyotype has not been reported yet. Here we report a case of esthesioneuroblastoma with a 47, XYY karyotype.
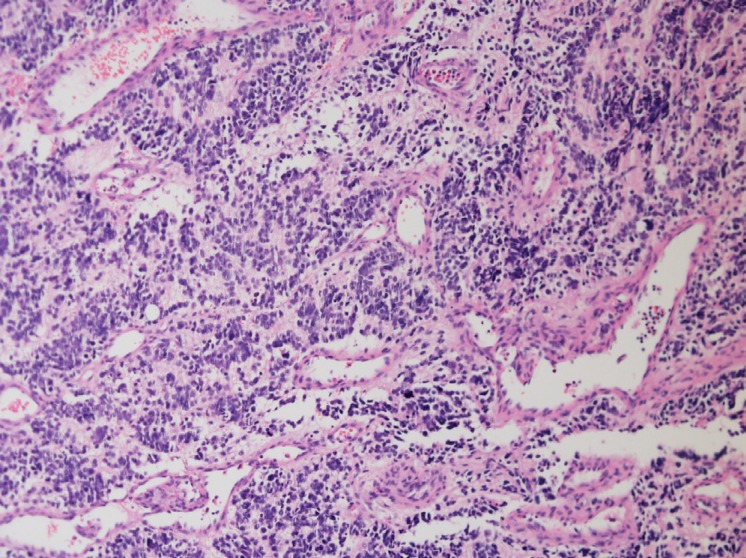
A 6-year-old male was admitted to the hospital with complaints of bilateral nasal obstruction and palpable cervical lymph node lasting several days. He was previously healthy boy with normal personality, and no familial history associated malignancy was detected. His height was 116 cm (50th percentile) and weight 22.7 kg (50th percentile). The physical examination revealed that he had enlarged cervical lymph node without tenderness, and hepatosplenomegaly was not remarkable. The polypoid mass lesion in right nasal cavity was detected via sinuscopy. Initial complete blood cell count showed white blood cell count 11,000/┬ĄL, hemoglobin 12.5 g/dL, and platelet 363,000/┬ĄL. Serum lactate dehydrogenase was 228 IU/L, serum ferritin was 55.2 mcg/L, and serum neuron-specific enolase level was increased 50.9 ng/mL. Urinalysis revealed normal levels of vanillylmandelic acid (7.56 mcg/mg creatinine), but slightly elevated levels of homovanillic acid (14.3 mcg/mg creatinine). The other laboratory findings were within normal limit. Magnetic resonance imaging (MRI) demonstrated lobulated nodular lesion on right posterior nasal cavity (Fig. 1). Biopsy of the right nasal polyp was performed and the patient was diagnosed as having esthesioneuroblastoma with high grade (Hyam's histologic grading III) (Fig. 2). N-myc gene amplification of primary tumor sample was not detected differences to normal gene. 18-Fluorodeoxyglucose positron emission tomography (FDG-PET) and I-123-Metaiodobenzyl-guanidine (MIBG) scintigraphy, evaluation tools of metastatic disease, showed that increased uptake both right posterior nasal cavity and right cervical IB-II space, suggested probably metastatic lymph nodes. In bone marrow examination, there were no disclosed blastic cells suggesting bone marrow involvement. Cytogenetic analysis with bone marrow preparation was performed after a 48-hour culture without stimulation, and demonstrated a 47, XYY karyotype in somatic cells (Fig. 3). The patient was diagnosed with esthesioneuroblastoma with high risk group according to Children's Oncology Group Risk Group Assignment for Neuroblastomab12); advanced age (Ōēź547 days), unfavorable histology, and cervical lymph node metastasis demonstrated by FDG-PET (International Neuroblastoma Staging System, stage 3).
Chemotherapy was performed alternately with two regimens; modified CCG 321P2 regimen (cisplatin 60 mg/m2 on day 0, etoposide 100 mg/m2 on day 3 and day 6, doxorubicin 30 mg/m2 on days 3, and cyclophosphamide 30 mg/kg on day 4 and day 5) and modified high-dose ifosfamide, carboplatin and etoposide regimen (carboplatin 400 mg/m2 on day 0 and day 1, etoposide 100 mg/m2 on days 0 to 4, and ifosfamide 1,200 mg/m2 on days 0 to 4). Chemotherapy was conducted until the 12th chemotherapy session. MRI and I-123-MIBG scintigraphy was performed during the first year of chemotherapy. After 3rd chemotherapy session, abnormal accumulations, initially seen at the primary tumor site and right cervical lymph nodes, were not observed. We did not perform surgical treatment and autologous stem cell transplantation after the 6th chemotherapy due to complete remission of disease. Radiotherapy was not conducted due to possibility of facial asymmetry at the age of the patient. After completion of 12th chemotherapy session, the patient had no disease recurrence after 3 years, and he is still under follow-up.
In treatment period, he manifested behavioral violence and temper tantrum. He also seemed that he unfocused and distracted any time, as it is already known in XYY syndrome. Then, we referred psychological consultation for management of behavior disorder and started methylpenidate. He still has behavioral inattentiveness and hyperactivity, so he is treated with methylpenidate for correction of behavior and monitored at outpatient clinic.
Several reports of constitutional chromosome anomalies in children presenting with malignancies have been described in the past to identify important genes related the development of pediatric malignancies. Reports regarding constitutional chromosome anomalies in children with neuroblastoma still remained sporadic. Satge et al.10) reported constitutional karyotype anomalies associated with neuroblastoma and analyzed with previously published cases to identify genes involved in children with neuroblastoma. Moreover, they found that it seems to be different of aggressiveness of tumors in the several cytogenetic types, for example, less aggressive behavior in aneuploidies and balanced translocations, in contrast to more advanced stage in unbalanced aberration of chromosome10). But no case of neuroblastoma related 47, XYY karyotype was mentioned in this review.
The XYY syndrome may present with a wide variation of physical and behavioral clinical features. The commonest indications of karyotyping for diagnosis are developmental delay and/or behavioral difficulties. This sex-chromosome aneuploidy is not characterized by distinct physical features or recognizable pattern of neurodevelopmental characteristics. Psychological studies suggested that certain personality traits, such as infantilism, lack of emotional control, increased impulsiveness were so characteristic that patients with 47, XYY6). Our patient had characteristics as usually expected in XYY syndrome. Finally, psychosocial evaluation recognized mild developmental retardation and learning disabilities requiring educational support.
Several reports of hematologic malignancies in boys with 47, XYY appear to be coincidental due to the routine karyotyping in the diagnostic work-up6,13). Although an increased incidence of leukemia in Down syndrome, the association of other constitutional aberrations involving sex chromosomes with malignancies is a matter of current investigation1,4,7). The role of the Y chromosome in the pathogenesis of leukemia is also controversial13,14). However, some reports implies that leukemia is expected to be more frequently in males than in females15). The Y chromosome may have a growth-stimulating effect on the hematopoietic cells and other tissues of the body originating from mesoderm and associated with overgrowth of bones and teeth14). Neuroblastoma and other malignancies are rarely associated with XYY karyotype. Honma et al. reported one case of neuroblastoma in the XYY karyotype11). They could not also postulate the relation between the excessive Y chromosome and neuroblastoma with a one case. Krossnes et al. reported DNT associated with the XYY karyotype9). Reported studies of the relationship between XYY karyotype and malignancies are rare. However, further study should be needed for investigation of oncogenesis of XYY karyotype, if the patients were continuously detected.
Our patient had the XYY syndrome. This raises the question of whether this association is specific or coincidental. There is a lack of evidence of association between the XYY karyotype and development of any neoplasms. Therefore, we need to determine the influence of genetic association in neuroblastoma with constitutional chromosome abnormalities including the XYY karyotype. Routine chromosomal analysis in malignancies may be helpful for determination of association with constitutional chromosome abnormalities including XYY karyotype.
Notes
Conflict of interest:
No potential conflict of interest relevant to this article was reported.
References
1. Hecht F. Risks of hematologic malignancy with constitutional chromosome abnormalities. Cancer Genet Cytogenet 1987;24:375ŌĆō377.


2. Harnden DG, Maclean N, Langlands AO. Carcinoma of the breast and Klinefelter's syndrome. J Med Genet 1971;8:460ŌĆō461.



3. Hasle H, Jacobsen BB, Asschenfeldt P, Andersen K. Mediastinal germ cell tumour associated with Klinefelter syndrome: a report of case and review of the literature. Eur J Pediatr 1992;151:735ŌĆō739.


5. Xavier AC, Ge Y, Taub JW. Down syndrome and malignancies: a unique clinical relationship: a paper from the 2008 william beaumont hospital symposium on molecular pathology. J Mol Diagn 2009;11:371ŌĆō380.



6. Gravholt CH. Sex-chromosome abnormalities. Pyeritz RE, Rimoin DL, Korf BR, Emery AE, editors. Emery and Rimoin's principles and practice of medical genetics. 6th ed. San Diego: Elsevier Academic Press, 2013;:1038ŌĆō1057.
7. Welborn J. Constitutional chromosome aberrations as pathogenetic events in hematologic malignancies. Cancer Genet Cytogenet 2004;149:137ŌĆō153.


8. Benitez J, Valcarcel E, Ramos C, Ayuso C, Cascos AS. Frequency of constitutional chromosome alterations in patients with hematologic neoplasias. Cancer Genet Cytogenet 1987;24:345ŌĆō354.


9. Krossnes BK, Wester K, Moen G, Mork SJ. Multifocal dysembryoplastic neuroepithelial tumour in a male with the XYY syndrome. Neuropathol Appl Neurobiol 2005;31:556ŌĆō560.


10. Satge D, Moore SW, Stiller CA, Niggli FK, Pritchard-Jones K, Bown N, et al. Abnormal constitutional karyotypes in patients with neuroblastoma: a report of four new cases and review of 47 others in the literature. Cancer Genet Cytogenet 2003;147:89ŌĆō98.


11. Honma T, Kanegane H, Shima T, Otsubo K, Nomura K, Miyawaki T. Neuroblastoma in an XYY male. Cancer Genet Cytogenet 2006;168:83ŌĆō84.


12. Maris JM. The biologic basis for neuroblastoma heterogeneity and risk stratification. Curr Opin Pediatr 2005;17:7ŌĆō13.


13. Oguma N, Shigeta C, Kamada N. XYY male and hematologic malignancy. Cancer Genet Cytogenet 1996;90:179ŌĆō181.


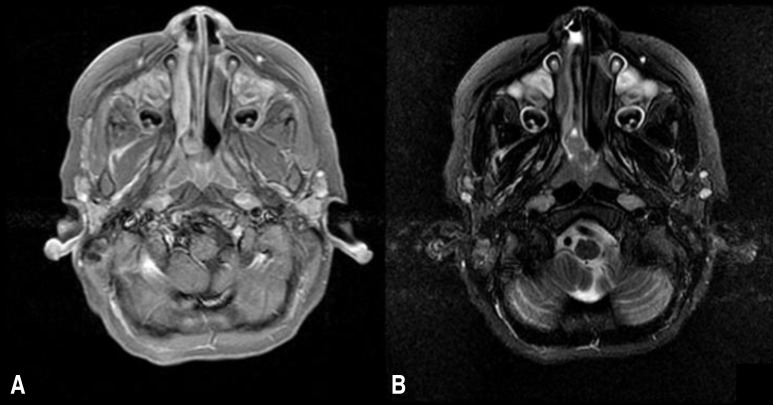
Fig.┬Ā1
Paranasal sinus (PNS) magnetic resonance imaging (MRI) of the patient. (A) A gadolinium-enhanced T1-weighted axial PNS MRI scan and (B) a T2-weighted axial PNS MRI scan showing a 1.2-cm lobulated nodule on the right posterior nasal cavity.




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link PubMed
PubMed Download Citation
Download Citation


