Article Contents
| Korean J Pediatr > Volume 61(7); 2018 |
|
Abstract
Purpose
The present study aimed to evaluate progression and prognosis according to the palliation method used in neonates and early infants aged 3 months or younger who were diagnosed with pulmonary atresia with ventricular septal defect (PA VSD) or tetralogy of Fallot (TOF) with severe pulmonary stenosis (PS) in a single tertiary hospital over a period of 12 years.
Methods
Twenty with PA VSD and 9 with TOF and severe PS needed initial palliation. Reintervention after initial palliation, complete repair, and progress were reviewed retrospectively.
Results
Among 29 patients, 14 patients underwent right ventricle to pulmonary artery (RV-PA) connection, 11 palliative BT shunt, 2 central shunt, and 2 ductal stent insertion. Median age at the initial palliation was 13 days (1–98 days). Additional procedure for pulmonary blood flow was required in 5 patients; 4 additional BT shunt operations and 1 RV-PA connection. There were 2 early deaths among patients with RV-PA connection, one from RV failure and the other from severe infection. Finally, 25 patients (86%) had a complete repair. Median age of total correction was 12 months (range, 2–31 months). At last follow-up, 2 patients had required reintervention after total correction; 1 conduit replacement and 1 right ventricular outflow tract (RVOT) patch enlargements.
Conclusion
For initial palliation of patients with PA VSD or TOF with severe PS, not only shunt operation but also RV-PA connection approach can provide an acceptable outcome. To select the most proper surgical strategy, we recommend thorough evaluation of cardiac anomalies such as RVOT and PA morphologies and consideration of the patient’s condition.
Patients with pulmonary atresia with ventricular septal defect (PA VSD) or tetralogy of Fallot (TOF) with severe pulmonary stenosis (PS) need pulmonary circulation through the ductus arteriosus or major aortopulmonary collateral arteries (MAPCAs). As a result, they require immediate prostaglandin E1 (PGE1) injection after birth and a palliative procedure within 1 week from birth for stable pulmonary blood flow [1,2].
Recently, early primary complete repair was introduced to reduce ventricular hypertrophy-mediated risks related to traditional palliative procedures in the TOF patient group, if the size of pulmonary artery (PA) would allow it [3,4]. Early primary repair provides avoidance of shunt-related complications, early relief of hypoxia, and normal lung development [5,6]. However, recent meta-analysis has reported that neonatal TOF repair was significantly associated with an increase in mortality, longer hospital stay, longer intensive care unit stay and a marked increase in the use of a transannular patch [7]. Therefore the method of performing palliative procedure first and then performing the complete repair procedure to reduce early postoperative mortality is still frequently used [7,8].
A traditional palliative procedure used in patients with PA VSD and TOF with severe PS is modified Blalock-Taussig (BT) shunt placement; however, stenosis or occlusion of the junction between the shunt and PA can be occurred. If the shunt is too big, heart failure may occur from excessive pulmonary blood flow, and diuretics may be required to treat the heart failure in such cases [9,10].
Recently, right ventricle to PA (RV-PA) connection was introduced instead of modified BT shunt placement. The advantages of the RV-PA connection procedure include the absence of aortic diastolic steal syndrome and the lower incidence of PA stenosis compared with that for BT stunt placement [11]. However, the procedure requires a cardiopulmonary bypass and ventriculotomy. Also, aneurysm due to right ventricular outflow tract (RVOT) patch may occur [12].
In addition, surgical method such as insertion of a central systemic to pulmonary shunt or procedure that preserve pulmonary flow by inserting a stent into the patent ductus arteriosus (PDA) are also being used.
The present study aimed to evaluate progression and prognosis according to the palliation method used in neonates and early infants aged 3 months or younger who were diagnosed with PA VSD or TOF with severe PS in a single tertiary hospital over a period of 12 years.
The present study retrospectively investigated patients diagnosed with PA VSD or TOF with severe PS at Kyungpook National University Hospital (KNUH) between January 2005 and December 2015. The following cases were excluded: (1) patients who had possibility of failure of 2 ventricle repair, (2) patients who did not need palliative surgery due to acceptable room air oxygen saturation.
Patients with atresia of both the pulmonary valve and a variable length of main PA (MPA) along with remaining three features of classic TOF (large, malaligment, membranous or subarterial type VSD, variable degree of aortic override, and RV hypertrophy) detected by echocardiography were diagnosed as PA VSD. PA VSD is divided into 3 types according to PA morphology, presence of PDA and MAPCA; type A, native PAs are present and pulmonary vascular supply through PDA and no MAPCAs; type B, native PAs are present and MAPCAs present; type C, native PAs are not present and pulmonary blood supply through MAPCAs only [13].
Patients who had classic TOF and PS such as infundibular hypertrophy, pulmonary valvular stenosis, or MPA hypoplasia, and showed extreme cyanosis of oxygen saturation <70% [1] were diagnosed as TOF with severe PS.
The period following the palliative procedure to complete repair was defined as interstage, while completion of treatment was defined as the point when the complete repair procedure was performed, regardless of when it was performed.
Nakata PA index is calculated from the diameter of PAs measured immediately proximal to the origin of upper lobe branches of the respective branch PAs [14] from cardiac computed tomography (CT). The sum of the cross sectional area (CSA) of right and left PAs is divided by the body surface area of the patient (Nakata index=CSA of right pulmonary artery [mm2]+CSA of left pulmonary artery [mm2]/BSA [m2]).
Medical records were accessed to investigate each patient’s birth information, genetic abnormality, age, hypoxia status at the time of admission, and heart anomaly diagnosed using initial cardiac CT or initial 2-dimensional echocardiography. Timing and method of palliation, structural anomalies of the heart that affected the decision on palliation method, progression following palliation, and reintervention status were examined for each patient and compared. Moreover, timing of surgery, surgical method, and long-term prognosis via outpatient follow-up observations were also investigated.
Search and use of patient information were reviewed and approved by the medical records department and Institutional Review Board (IRB) at KNUH (approval numbr: 2016-11-019). Written informed consent was waived by IRB.
Four types of palliation procedures were applied: modified BT shunt placement, RV-PA connection, central shunt placement, and PDA stent placement. Median sternotomy was applied in all surgical cases.
Modified BT shunt placement is a method in which a GORE-TEX Vascular Grafts (W. L. Gore & Associates, Inc., Flagstaff, AZ, USA) is used to connect the PA with the subclavian artery. RV-PA connection is a method that connects the remaining MPA tissue with the right ventricle using autologous or prosthetic material after median sternotomy. This included cases in which connection was combined with partial excision, if RVOT muscle hypertrophy was detected [8,15]. Central shunt placement is a method that connects the ascending aorta with MPA using an autologous vessel or prosthetic material, while PDA stent is a method that involves inserting a stent into the PDA, and when necessary, banding the PA to prevent pulmonary overcirculation [16].
The palliative procedure method was chosen based on the severity of heart pathology after consultation between a pediatric cardiologist and pediatric cardiac surgeon. A single surgeon performed all procedures.
All statistical analyses were performed with IBM SPSS Statistics ver. 23.0 (IBM Co., Armonk, NY, USA), and collected data were described as means with the standard deviations or median with the range. Univariate comparisons of continuous variables were conducted using the unpaired Student t test. Univariate analyses of the differences in proportion between the 2 groups were accomplished using a chi-square analysis. A P value of <0.05 was considered statistically significant.
A total of 29 patients were included in the present study: 20 with PA VSD and 9 with TOF with severe PS. The characteristics of patients are shown in Table 1.
The patient population included 18 boys and 11 girls. Their median gestational age was 38 weeks (range, 34–40 weeks). Five patients were premature infants (gestational age<37 weeks). The median birth weight was 2,740 g (range, 1,820–4,250 g). Genetic abnormalities were found in 6 patients, including 2 patients with Down syndrome, 1 female with 46XY external genitalia, 1 patient with chromosome seven anomaly (der(7;15)(p10;q10)), 1 patient with CATCH22 syndrome (del 22q11.2), and 1 patient with long QT syndrome (LQTS) family.
RVOT morphology of 20 patients with PA VSD included 14 cases of visible MPA and 6 cases of only a confluent PA without MPA tissue. There are no patients with MAPCAs (PA VSD types B and C). The median Nakata index value of patients with PA VSD was 87.96 mm2/m2 (range, 48.00–266.21 mm2/m2).
Among patients with TOF with severe PS, the cause of PS was severe infundibular hypertrophy in 5 patients, valvular PS in 1 patient, and MPA hypoplasia in 3 patients. The median Nakata index value of patients with TOF with severe PS was 58.72 mm2/m2 (range, 39.58–111.91 mm2/m2).
The median Nakata index value was not significantly different between patients with PA VSD and patients with TOF with severe PS (P=0.056).
A total of 13 patients had hypoxia with oxygen saturation<70% despite using PGE1, including 6 patients with PA VSD and 7 patients with TOF with severe PS.
The median age at the time when palliative procedure was performed was 13 days (range, 1–98 days) in the PA VSD group and 24 days (range, 9–51 days) in the TOF with severe PS group, showing no statistically significant difference (P=0.176). The palliation procedures of each group are shown in Table 1.
RV-PA connection was used as the palliative procedure in 14 patients, including 9 patients from PA VSD group and 5 patients from TOF with severe PS group, with the median age at the time of palliation being 19.5 days (range, 7–69 days). The median Nakata index value at the time of RV-PA connection was 62.88mm2/m2 (range, 39.58-300.80mm2/m2). Infundibulectomy was performed together with the palliative procedure in 2 patients who underwent RV-PA connection (Table 2). One patient with PA VSD who underwent RV-PA connection was transferred from another hospital. At birth, this patient showed a relatively large PA size on echocardiogram with intact PDA and acceptable oxygen saturation. But, aggravated hypoxia and poor oral intake developed from 50 days after birth, RV-PA connection was performed on 69 days after birth. Except for this patient, the median age at the time of RV-PA connection was 15 days (range, 7–26 days).
Modified BT shunt placement was used as the palliative procedure in 11 patients, including 7 patients from the PA VSD group and 4 patients from the TOF with severe PS group, with the median age at the time of palliation of 16.5 days (range, 1–98 days). The median Nakata index value at the time of modified BT shunt was 89.75 mm2/m2 (range, 50.38–266.20 mm2/m2). Infundibulectomy was performed together with the palliative procedure in 2 patients who underwent BT shunt (Table 2).
There were no significant differences of age of initial palliation and Nakada index between patients with RV-PA connection and patients with BT shunt (P=0.298 and P=0.913, respectively)
Two patients underwent central shunt placement and 2 patients underwent PDA stent placement as the palliative procedure (Table 1).
Among 20 patients in the PA VSD group, 1 patient was transferred to another hospital prior to complete repair, and 1 patient was lost to follow-up after undergoing BT shunt placement as the palliative procedure (Fig. 1). One patient died and the cause of death was sepsis caused by enterococci at 2 months after RV-PA connection. Five patients required additional palliative procedures due to unstable oxygen saturation. The first and second patients who underwent RV-PA connection as the initial palliation underwent modified BT shunt placement on day 8 and 12 after the initial procedure. The third patient underwent additional BT shunt placement on the left side 3 days after right modified BT shunt. The fourth patient who underwent PDA stent developed thrombus in the stent underwent RV-PA connection and PA banding 1 day after the initial palliation. The fifth patient who had an obstruction in the distal portion of the PDA originating from the right aortic arch underwent BT shunt placement using PDA underwent central shunt placement 6 days after the initial palliation due to shunt stenosis.
Among 9 patients in the TOF with severe PS group, 1 patient died 2 days later from the operation day of RV-PA connection (Fig. 2). No patient in the TOF with severe PS group required reintervention.
Two early dead patients underwent RV-PA connection as the initial palliation, whereas no death occurred among patients who underwent modified BT shunt placement (Fig. 1).
There were no significant differences between patients with PA VSD and patients with TOF with severe PS in the occurrence of heart failure/hypoxia, the need of additional palliation, and early death (P=0.697, P=0.649, P=0.379, and P=0.303, respectively).
In the PA VSD group, complete repair procedure was performed in 17 patients. Among of them, 11 and 6 patients underwent Rastelli operation and RVOT reconstruction via transannular patch (TAP), respectively.
In the TOF with severe PS group, complete repair procedure was performed in 7 patients. Among them, 6 patients underwent RVOT reconstruction with TAP and 1 patient underwent reconstruction without TAP.
Among the total of 29 patients, 25 patients received complete repair. A total of 11 patients underwent complete repair procedure subsequent to RV-PA connection, and the median age for complete repair was performed was 11.8 months (range, 2.4–30.7 months). A total of 10 patients underwent the complete repair procedure subsequent to modified BT shunt placement and the median age for complete repair was performed was 13.5 months (range, 5.9–18.3 months). The median age for complete repair was significantly different between PA VSD and TOF with severe PS (P=0.047), but not significantly different between RV-PA connection and BT shunt (P=0.888).
One patient died among 25 patients who had undergone complete repair. That patient belonged to the TOF with severe PS group and had undergone RV-PA connection as the palliative procedure. The patient, who died during sleep at 20-months-old, had a family history of LQTS, presented with bradycardia since neonate stage, and required long-term inpatient treatment for RV failure after complete repair.
Currently, there are 24 patients who have survived. Among them, one patient with PA VSD who had undergone complete repair subsequent to RV-PA connection ended up undergoing RVOT widening and LPA OS angioplasty 7 months later. Another patient with PA VSD underwent valved conduit change 5 years after the complete repair procedure. Besides these 2 patients, other patients are undergoing long-term outpatient follow-up and echocardiography examinations without any specific complications.
The results of the present study showed that RV-PA connection can be done for PA VSD or TOF patients with small Nakada index and that they did not need more management after initial palliation in comparison to BT shunt.
Modified BT shunt placement is one of the systemic to pulmonary shunt methods that connects the innominate or subclavian artery to ipsilateral PA with a synthetic interposition graft. It is a traditional palliative procedure used prior to complete repair for maintaining proper pulmonary blood flow and PA growth in cyanotic heart disease group, including PA VSD and TOF with severe PS [4].
Although modified BT shunt placement is still widely used for patients with ductal dependent pulmonary circulation, various studies have reported the disadvantages of modified BT shunt placement. It may increase the incidence of hypoplasia and distortion in the adjoining PA, which can lead to PA stenosis or occlusion, and when the shunt size is too big, excessive pulmonary blood flow can increase the probability of heart failure, which may require the use of diuretics or other medications to treat heart failure [4,5]. In addition, overshunting and shunt thrombosis can increase interstage mortality between BT shunt operation and complete surgical repair [5,6], with interstage mortality for infants reported to range between 10% and 15% [11].
Meanwhile, RV-PA connection, which is used for RVOT reconstruction by connecting the right ventricular infundibulum and central PA with prosthetic material or autologous/heterologous tissue, is also being used as an alternative that can address the shortcomings of modified BT or central shunts. RV-PA connection has the advantages of not being restricted in position setting even with abnormal aortic arch branching and being able to avoid aortic diastolic steal syndrome that can occur from decreased coronary artery perfusion pressure due to pulmonary blood flow [6]. Theoretically, RV-PA connection does not require anticoagulation and has lower risk of hemolysis or shunt kinking [11]. Moreover, because the RV-PA shunt has a diameter that is bigger than that of BT shunt, it also has the advantage of lower incidences of shunt occlusion [6].
RV-PA connection offers greater benefits with regard to postoperative PA growth than other methods, because balanced distribution of pulsatile blood flow from the RV to the PA has a more positive effect on PA growth. Some reports indicated that Norwood procedure group, which used RV-PA conduit, showed greater PA growth than the group that underwent systemic to pulmonary shunt placement [17,18]. Other cohort study reported that the RV-PA connection group showed favorably higher complete repair rate than the systemic-pulmonary shunt placement group, including BT shunt placement [11]. However, RV-PA connection has the disadvantage of requiring the use of a cardiopulmonary bypass and ventriculotomy during surgery, and in rare cases, aneurysm due to RVOT patch may occur [6,7,10].
In the present study, there were no significant differences in interstage morbidity and mortality rates and reintervention rates according to the used palliative procedure.
There are cases in which RV-PA connection may be difficult due to structural issues [1]; cases with anomaly that involves the left anterior descending coronary artery originating from the right coronary ostia or right coronary artery and pass through the infundibulum; cases with severe hypoplasia of the native PA. In such cases, the distance between the RV infundibulum and native PA is increased, which make stable correction difficult due to the need to pull the PA more excessively and more conduits are necessary during RV-PA connection; cases where sufficient space cannot be created via surgery due to the RVOT being filled with muscle. In the present study, no patient who underwent RV-PA connection had coronary artery anomaly. All 9 patients in the PA VSD group who underwent RV-PA connection had adequate native MPA size, while one patient who had muscular blockage of the RVOT underwent modified BT shunt placement. Among 7 patients in the PA VSD group who underwent modified BT shunt placement, 4 patients showed absence or hypoplasia of MPA. Among 5 patients in the TOF with severe PS group who underwent RV-PA connection, 2 patients had severe infundibular hypertrophy, for which infundibulectomy was also performed during surgery.
Palliative procedure is usually performed at 7–10 days after birth to maintain adequate pulmonary flow and for rehabilitation of diminutive PA. The reason the procedure is not performed upon birth is because the patient needs time to adapt to the transition from postnatal fetal circulation to adult circulation [12].
In previous studies enrolled patients with TOF or PA VSD, the mean age of RV-PA connection was 9–10 days [1]. In the present study, the mean age of patients who underwent RV-PA connection was 21.4 days, and if we excluded 1 patient who was born prematurely and needed to wait an appropriate time for surgery, the mean age was 15 days. The reason for the present study showing difference in the time of surgery as compared with other studies was due to additional time taken to increase the body weight of patients to perform RV-PA connection over BT shunt placement.
The interval between palliation and complete surgical repair was significantly shorter in the RV-PA connection group than in the systemic to pulmonary shunt group. In a previous study, the interval between palliative procedure and complete repair surgery was shorter in the RV-PA connection group (11.8 months) than in the systemic-pulmonary shunt group (16.8 months) [19]. The present study also confirmed that the RV-PA connection group had a shorter interstage duration until complete repair than the BT shunt group.
The limitations in the present study included a retrospective study and no definite control group. Statistical comparison between the PA VSD and TOF with severe PS groups was difficult due to small size of the patient population.
In conclusion, for initial palliation of patients with PA VSD or TOF with severe PS, not only palliative BT shunt operation but also RV-PA connection approach can provide an acceptable outcome. To select the most proper surgical strategy, we recommend thorough evaluation of cardiac anomalies such as RVOT and PA morphologies and consideration of the patient’s condition.
Fig. 1.
Interstage course between palliation and complete repair of patients with pulmonary atresia and ventricular septal defect (PA VSD). RV-PA, right ventricle-pulmonary artery; BT shunt, Blalock-Taussig shunt; F/U, follow-up; PDA, patent ductus arteriosus.
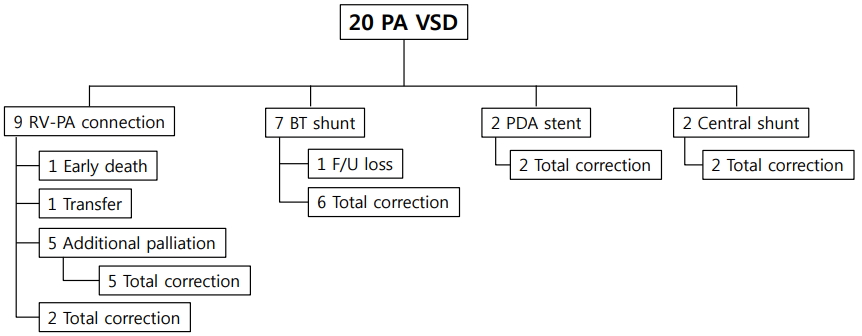
Fig. 2.
Interstage course between palliation and complete repair of patients with tetralogy of Fallots and severe pulmonary stenosis (TOFPS). RV-PA, right ventricle-pulmonary artery; BT shunt, Blalock-Taussig shunt.
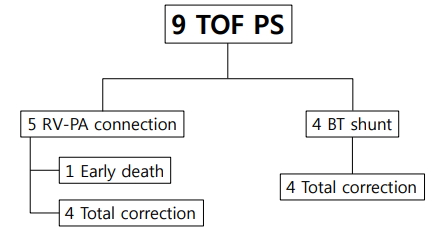
Table 1.
Patient characteristics
Values are presented median (range) or number.
PA VSD, pulmonary atresia with ventricular septal defect; TOF, tetralogy of Fallot; PS, pulmonary stenosis; BT shunt, Blalock-Taussig shunt; RV-PA, right ventricle-pulmonary artery; PDA, patent ductus arteriosus; LQTS, long QT syndrome; BSA, body surface area; RPA, right pulmonary artery; LPA, left pulmonary artery.
Table 2.
A comparative data; RV-PA connection and modified BT shunt
References
1. Gerelli S, van Steenberghe M, Murtuza B, Bojan M, Harding ED, Bonnet D, et al. Neonatal right ventricle to pulmonary connection as a palliative procedure for pulmonary atresia with ventricular septal defect or severe tetralogy of Fallot. Eur J Cardiothorac Surg 2014;45:278–88.



2. Villafañe J, Lantin-Hermoso MR, Bhatt AB, Tweddell JS, Geva T, Nathan M, et al. D-transposition of the great arteries: the current era of the arterial switch operation. J Am Coll Cardiol 2014;64:498–511.



3. Balaguru D, Dilawar M. Pulmonary atresia with ventricular septal defect: systematic review. Heart Views 2007;8:52–61.
4. Kim H, Sung SC, Chang YH, Jung W, Lee HD, Park JA, et al. Outcome of staged repair of tetralogy of Fallot with pulmonary atresia and a ductus-dependent pulmonary circulation: should primary repair be considered? Korean J Thorac Cardiovasc Surg 2011;44:392–8.



5. Pozzi M, Trivedi DB, Kitchiner D, Arnold RA. Tetralogy of Fallot: what operation, at which age. Eur J Cardiothorac Surg 2000;17:631–6.



6. Jonas RA. Early primary repair of tetralogy of Fallot. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu 2009;39–47.


7. Loomba RS, Buelow MW, Woods RK. Complete repair of tetralogy of Fallot in the neonatal versus non-neonatal period: a meta-analysis. Pediatr Cardiol 2017;38:893–901.



8. Dragulescu A, Kammache I, Fouilloux V, Amedro P, Métras D, Kreitmann B, et al. Long-term results of pulmonary artery rehabilitation in patients with pulmonary atresia, ventricular septal defect, pulmonary artery hypoplasia, and major aortopulmonary collaterals. J Thorac Cardiovasc Surg 2011;142:1374–80.


9. Gladman G, McCrindle BW, Williams WG, Freedom RM, Benson LN. The modified Blalock-Taussig shunt: clinical impact and morbidity in Fallot's tetralogy in the current era. J Thorac Cardiovasc Surg 1997;114:25–30.


10. Singh SP, Chauhan S, Choudhury M, Malik V, Talwar S, Hote MP, et al. Modified Blalock Taussig shunt: comparison between neonates, infants and older children. Ann Card Anaesth 2014;17:191–7.


11. Bradley SM, Erdem CC, Hsia TY, Atz AM, Bandisode V, Ringewald JM. Right ventricle-to-pulmonary artery shunt: alternative palliation in infants with inadequate pulmonary blood flow prior to two-ventricle repair. Ann Thorac Surg 2008;86:183–8.


12. Choi KH, Sung SC, Kim H, Lee HD, Ban GH, Kim G, et al. Right ventricle-to-pulmonary artery shunt in pulmonary atresia with a ventricular septal defect: a word of caution. Pediatr Cardiol 2017;38:707–11.



13. Tchervenkov CI, Roy N. Congenital heart surgery nomenclature and database project: pulmonary atresia--ventricular septal defect. Ann Thorac Surg 2000;69(4 Suppl): S97–105.


14. Nakata S, Imai Y, Takanashi Y, Kurosawa H, Tezuka K, Nakazawa M, et al. A new method for the quantitative standardization of cross-sectional areas of the pulmonary arteries in congenital heart diseases with decreased pulmonary blood flow. J Thorac Cardiovasc Surg 1984;88:610–9.


15. Fouilloux V, Bonello B, Kammache I, Fraisse A, Macé L, Kreitmann B. Management of patients with pulmonary atresia, ventricular septal defect, hypoplastic pulmonary arteries and major aorto-pulmonary collaterals: Focus on the strategy of rehabilitation of the native pulmonary arteries. Arch Cardiovasc Dis 2012;105:666–75.


16. Yuan SM, Jing H. Palliative procedures for congenital heart defects. Arch Cardiovasc Dis 2009;102:549–57.


17. Caspi J, Pettitt TW, Mulder T, Stopa A. Development of the pulmonary arteries after the Norwood procedure: comparison between Blalock-Taussig shunt and right ventricular-pulmonary artery conduit. Ann Thorac Surg 2008;86:1299–304.







 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link PubMed
PubMed Download Citation
Download Citation


