Article Contents
| Korean J Pediatr > Volume 61(11); 2018 |
|
Abstract
Acute kidney injury (AKI) is characterized by abrupt deterioration of renal function, and its diagnosis relies on creatinine measurements and urine output. AKI is associated with higher morbidity and mortality, and is a risk factor for development of chronic kidney disease. There is no proven medication for AKI. Therefore, prevention and early detection are important. Physicians should be aware of the risk factors for AKI and should monitor renal function in high-risk patients. Management of AKI includes optimization of volume status and renal perfusion, avoidance of nephrotoxic agents, and sufficient nutritional support. Continuous renal replacement therapy is widely available for critically ill children, and this review provides basic information regarding this therapy. Long-term follow-up of patients with AKI for renal function, blood pressure, and proteinuria is recommended.
Acute kidney injury (AKI), previously known as acute renal failure, indicates abrupt deterioration of renal function. While acute renal failure often implies anuria and urgent need of emergency renal replacement therapy (RRT), AKI is a broader term coined to detect this condition at an early stage, thereby improving its outcome through early intervention. AKI occurs in about 60% of patients in the intensive care unit (ICU). In fact, even early-stage AKI is proven to be associated with higher morbidity and mortality (Fig. 1) [1], especially in critically ill patients. In addition, there is mounting evidence against the previous notion that no long-term sequelae can occur after complete resolution of acute renal failure; AKI is a risk factor for chronic kidney disease (CKD) [1]. Therefore, timely detection of and intervention for AKI are necessary to improve the short-term outcome of this condition and to prevent CKD.
Authors of various studies previously used their own definitions of AKI, which have hindered appropriate collective studies on AKI. In 2012, Kidney Disease: Improving Global Outcomes (KDIGO) announced a new definition of AKI, summarizing the previously widely accepted criteria (Table 1) [2]. For neonates, modification of criteria of absolute creatinine level for stage III AKI was proposed as 2.5 mg/dL [3].
However, the definition of AKI based on serum creatinine level has several limitations. Serum creatinine is a functional marker of the kidney; its level increases hours or days after renal injury. Creatinine is dependent on muscle mass; fluid overload might dilute the serum creatinine concentration, and its clearance may be altered with the drug cimetidine or other organic molecules, as well as in systemic diseases. Notably, cystatin C, an inhibitor of cysteine proteinases from all nucleated cells [4], irrespective of muscle mass and age, is considered as an alternative for creatinine in estimating renal clearance. Glomerular filtration rate estimation equation using cystatin C was more accurate than that using creatinine [5]. However, cystatin C is affected by diabetes, high-dose corticosteroids, hyperthyroidism, inflammation, hyperbilirubinemia, rheumatoid factor, and hypertriglyceridemia [6].
Biomarkers of kidney injury, namely tubular injury markers of N-acetyl-β-D-glucosaminidase (proximal tubular damage marker), neutrophil gelatinase-associated lipocalin (approved in Korea and other countries), kidney injury molecule-1, liver fatty acid-binding protein (approved in Japan), and kidney stress and cell cycle arrest marker TIMP-2 & IGFBP-7 (NephroCheck [Astute Medical, San Diego, CA, USA], approved in USA) [7,8], have been investigated and validated. However, these are not yet widely used in clinics [9].
Once AKI is detected, a physician should determine its causes and manage them accordingly. Dehydration or decreased renal perfusion is the most common cause of AKI, followed by drug or nephrotoxininduced AKI [10,11]. Glomerulopathies including hemolytic uremic syndrome and all kinds of glomerulonephritis often accompany AKI (Table 2) [6,12,13].
A type of infection-induced acute tubulointerstitial disease is noteworthy. When a child presents with prolonged fever of unknown origin followed by gastrointestinal symptoms of abdominal pain and diarrhea, leading to a diagnosis of AKI, Yersinia pseudotuberculosis infection should be considered [14]. Y. pseudotuberculosis infection is spread by consumption of unsanitized water, and it may be complicated by AKI, which is self-limiting. It often resembles Kawasakilike disease, with strawberry tongue, desquamation of the fingertips, and dilatation of coronary arteries (Fig. 2) [14].
There is no proven medication for AKI. Therefore, prevention and early detection is the mainstay of management of AKI.
In general, dehydration; advanced age or prematurity; chronic diseases, especially CKD, diabetes, hypertension, cancer, anemia, and proteinuria in adults render the host susceptible to AKI [15]; volume depletion, sepsis, critical illness, cardiopulmonary bypass, ICU care, extracorporeal membrane oxygenation, surgery, ventilation, vasopressor usage, and exposure to nephrotoxins lead to an increased risk of AKI (Table 3) [2,16].
Every condition associated with AKI has its own risk factors; therefore, physicians should be aware of these risk factors for their disease of interest and should carefully monitor renal function in patients at risk of AKI [10]. For example, pediatric cardiac surgery; cyanotic heart disease; long extracorporeal circulation; higher risk according to the RACHS-1 (risk-adjusted classification for congenital heart surgery); postoperative low cardiac output syndrome; pump failure; sepsis; hematologic complications; preoperative dependency on the ventilator; treatment with milrinone, gentamicin, or furosemide; duration of anesthesia; use of multiple cross-clamps; and transfusion have been reported as risk factors for AKI [17,18]. A kidney scoring system called renal angina index was proposed to predict AKI in critically ill children [16]. It predicted AKI on the basis of subtle kidney injury (change of estimated creatinine clearance, or fluid overload) and patient risk factors (ICU admission, stem cell transplantation, ventilation and inotropy) [16].
Adequate renal perfusion is essential to prevent AKI and to recover from it. Optimal blood pressure and intravascular volume of the patient should be maintained (mean arterial pressure>65 mmHg or higher for a previously hypertensive host, central venous pressure 8–12 mmHg for an adult). Fluid resuscitation is initially necessary for patients with hypotension, if the hypotension is partially due to hypovolemia; however, fluid overload (weight gain more than 10%) in critically ill patients is associated with a poor outcome; therefore, fluid administration should be cautiously performed. Meanwhile, as long as the intravascular volume is adequate, inotropics should be considered to ensure adequate (renal) circulation [19-24].
With regard to the choice of resuscitation fluid, studies have shown that patients treated with colloid solutions such as hydroxyethyl starches show poorer outcomes compared to outcomes in patients treated with normal saline. Albumin seems not inferior to saline, except in patients with traumatic brain injury [6,25]. Some studies reported that normal saline leads to poorer outcomes than those observed using a balanced salt solution such as Ringers lactate solution. As normal saline is hyperchloremic, it could induce hyperchloremic metabolic acidosis, leading to decreased renal cortical tissue perfusion [26]. However, recent prospective studies failed to prove the efficacy of balanced solution over that of normal saline [27,28].
Antibiotics such as aminoglycoside, vancomycin (especially when used along with piperacillin/tazobactam) [6], amphotericin, nonsteroidal anti-inflammatory drugs (NSAIDs), angiotensinogenconverting enzyme inhibitor, angiotensin receptor blocker, calcineurin inhibitors, and chemotherapeutic agents such as cisplatin and methotrexate are well-known nephrotoxins. These nephrotoxins should be avoided in patients at risk of AKI and in those with established AKI. If the use of these agents is unavoidable, their dosage or dosing interval should be adjusted to reduce renal toxicity. For example, a once daily dose of aminoglycoside is less nephrotoxic than other regimens. In addition, the trough level should be measured and adjusted accordingly [29].
Contrast media used for angiography and computed tomography is nephrotoxic as well. Patients with CKD, diabetes, insufficient circulating blood volume, and those who take NSAIDs are at risk of contrast-induced AKI. Contrast dosage, type, and multiple procedures increase the risk of this condition. Minimization of contrastmedia volume, use of low-osmolar and iso-osmolar contrast media, and administration of noniodinated contrast media are recommended to prevent contrast-induced AKI [30]. Preventive measures include intravenous administration of an isotonic normal saline or Hartmann solution at 1 mL/kg/hr 12 hours before and after the procedure, or 3 mL/kg/hr 1 hour before and 6–9 mL/kg over 4–6 hours after the procedure [6,30]. KDIGO guidelines suggest using N-acetylcysteine in patients at increased risk of contrast-induced AKI [30], while a recent randomized controlled trial failed to show any benefit of N-acetylcysteine [31]. In addition, nephrotoxins should be discontinued more than 24 hours before the procedure.
As AKI is a catabolic status, nutritional support is necessary; oral nutrition is preferred.
- Plasma glucose 110–149 mg/dL (caution should be exercised, as patients with AKI are at risk of hypoglycemia)
- Total energy intake of 20–30 kcal/kg/day in patients at any stage of AKI (no restriction!)
- Protein 0.8–1.0 (1.5) g/kg/day in AKI (no RRT), 1.0–1.5 (3–4) g/kg/day on RRT, up to 1.7 g/kg/day on continuous RRT (CRRT)
Diuretics are effective only for preventing/ameliorating fluid overload and not for maintaining urine output volume to avoid AKI. Diuretics are not recommended to prevent AKI or to treat established AKI [33]. Mannitol, dopamine (other than as an inotropic) [34], glucocorticoid [35], fenoldopam [36], and N-acetylcysteine [37,38] (other than contrast-induced AKI) are also not recommended.
RRT is indicated when metabolic derangement of AKI, such as hyperkalemia or acidosis, and/or fluid overload cannot be managed with medications, or for detoxification. Some researchers recommend early initiation of RRT, but available data show mixed results in this regard [39,40]. Fluid overload of more than 10% of the body weight and inability to maintain input–output balance owing to large amount of fluid to treat the patients and/or oliguria are the common indications for RRT [41].
Intermittent hemodialysis (IHD), CRRT, and peritoneal dialysis (PD) are available as RRT for AKI. The choice of modality depends on availability at the center and the condition of the patient. IHD is possible only when the patient is hemodynamically stable, and when HD machine appropriate for the size of the patient, and dedicated personnel (pediatric nephrologist and dialysis nurses capable of performing pediatric IHD) are available. CRRT is a low-dose long-duration RRT; therefore, patients who are hemodynamically less stable can be managed with CRRT, usually in the ICU setting. However, it still requires appropriate machines suitable for CRRT in children. Although PD can be applied irrespective of the patient size, its dosage in critically ill patients is highly unpredictable, because dialysis is dependent on the peritoneal circulation. In addition, the risk of catheter-related infections is higher with PD, with a high risk of peritoneal fluid leakage through the PD catheter insertion site.
Recently, CRRT has become widely available for critically ill patients. Therefore, the following sections will focus on CRRT. In fact, several studies have shown that CRRT is superior to IHD for recovery from AKI, as interdialytic hypotension is more common in IHD than in CRRT.
In children, adequacy of CRRT (and IHD) often depends on the adequacy of vascular access, which determines maximum blood flow rate (BFR) and filter lifespan [41]. The shortest and largest possible catheter for the patient are recommended for successful CRRT, which ensures lower resistance and better blood flow (Table 4). For AKI, dual or triple lumen catheter dedicated to RRT or 2 single lumen catheters are used. On the contrary, the smallest catheter to facilitate appropriate BFR is recommended to protect the vessel of the patient. The locations of vascular access are the internal jugular vein and femoral vein; subclavian vein should be avoided to prevent stenosis of ipsilateral arm veins. Complications of vascular access such as infection (high chance in femoral vein); thrombosis; stenosis; and problems of catheter itself such as malfunction, kinking of the catheter, and recirculation between input lumen and output lumen should be considered when choosing vascular access.
Once vascular access for dialysis is secured, the CRRT kit to be used should be determined based on patient weight. A filter/membrane with a surface area closest to the body surface area (BSA) of the patient should be chosen (Table 5). The extracorporeal blood volume necessary to use the respective circuit should be known; allowable extracorporeal volume is less than 8 mL/kg (<10%–20% of the plasma volume). The difference between required circuit volume and allowable extracorporeal volume can be filled by priming the circuit with blood (blood priming) or 5% albumin.
(Ultra)filtration (hemofiltration, HF) uses convection to remove fluid and its solute across the membrane by a transmembrane (hydrostatic) pressure gradient that is exerted by the pump of the CRRT machine; the removed fluid is replaced by adding “replacement fluid” through the prefilter or postfilter port of the CRRT circuit. Hemodialysis (HD) uses diffusion of solute between blood (plasma) and dialysate (flowing in a direction opposite to that of the plasma to maximize the solute concentration difference) through filter (membrane) by the concentration difference. Convection can remove larger molecules than diffusion does; therefore, if the target molecule is large, HF is the preferred mode of CRRT. With continuous venovenous hemodiafiltration (CVVHDF), HD and HF are applied simultaneously; their ratio can be decided according to the condition of the patient. If the target molecule is small, such as that of ammonia, the ratio of HD can be increased for faster removal of the molecule.
Patients who require CRRT often have severe AKI with hyperphosphatemia and hyperkalemia. Therefore, the components of CRRT fluid are designed to treat these metabolic derangements (Table 6). In case of normal phosphorous/potassium levels, the CRRT fluid of the patient needs to be supplemented with additional phosphorous/potassium. Commercialized CRRT fluids containing additional phosphorous/potassium are available.
Creatinine clearance by CRRT is assumed to be equal to the effluent rate, as the creatinine concentration of the effluent from dialysis is equal to the serum creatinine (as long as the dialysis membrane is functional, because BFR is much higher than the counter-current dialysate flow rate [DFR]), and that of the effluent of ultrafiltration is equal to that of plasma (excluding large molecules that cannot pass the filtration). Although the optimal intensity of RRT is debatable, a filtration rate of 20–35 mL/hr/kg (≅20–35 mL/min/1.73 m2=1,200–2,100 mL/hr/1.73 m2) is considered sufficiently high [42,43]; in this way, effluent volume is calculated. Then, the effluent rate is ideally equal to the sum of the DFR and replacement fluid flow rate (RFFR), the ratio of which is set by the physician. When HD:HF=1:1, DFR and RFFR can be set as 10–17.5 mL/hr/kg. In practice, the delivered CRRT may not be equal to the prescribed dose; therefore, prescription should be 120%–125% of the computed clearance [41].
When higher clearance is required, such as in hyperammonemia, DFR can be set to more than 2,100 mL/hr/1.73 m2.
A minimum BFR of 30–50 mL/min is necessary for CRRT; the BFR should be up to 400 mL/min/BSA 1.73 m2 (250 mL/min), or 10–12 mL/kg/min for neonates and infants (weight<5 kg), 5–10 mL/kg/min in children (weight<30 kg), and 2–5 mL/kg/min for older children (weight≥30 kg). BFR is supposed to be 5–10 fold higher than DFR or RFFR. Ultrafiltrate (removed fluid from the plasma) should be less than 20% of the plasma; therefore, plasma flow rate (BFR×[1-hematocrit]) should be more than 5 times the ultrafiltration rate. Ultrafiltration rate is the sum of RFFR (which is removed as convection) and patient removal rate (PRR, [total input−total output+desired fluid removal]/24). Therefore, BFR should be larger than (5×[RFFR+PRR]/60 min)/(1−hematocrit).
Anticoagulation is necessary to maintain extracorporeal circulation. Systemic anticoagulation with heparin (100 units/mL solution, 10–25 units/kg as loading and 5–10 units/kg/hr as maintenance) is most commonly performed; however, bleeding-related complications are common, and heparin-induced thrombocytopenia is also a concern. Nafamostat mesylate (Futhan [SK chemicals, Seoul, Korea], 0.1–1 mg/kg/hr) is a short-acting (half-life, 5–8 minutes) agent associated with fewer bleeding-related complications than heparin is. However, regardless of whether heparin or nafamostat mesylate is used, activated clotting time should be monitored regularly. Anticoagulation-free, intermittent saline flushing can be performed for patients with a higher risk of bleeding.
When urine output recovers to >400 mL/day in adults and renal creatinine clearance recovers to >20–35 mL/min/1.73 m2, CRRT can be discontinued [44].
(1) Secure vascular access for dialysis
(2) Decide the filter/membrane of CRRT considering the BSA of the patient, and extracorporeal volume (<8 mL/kg) vs. membrane surface area (≅BSA) and circuit volume (blood/albumin priming, if necessary)
(3) Choose the CRRT fluid considering phosphorous/potassium concentration
(4) Decide the mode of CRRT; CVVHDF (HD+HF), CVVHD ([continuous venovenous hemodialysis], HD only), CVVHF ([continuous venovenous hemofiltration], HF only)
(5) Decide the intensity of CRRT. Effluent flow rate=20–35 mL/hr/kg
(6) Calculate BFR (30–50 mL/min to ≤400 mL/min/1.73 m2)
- 10–12 mL/kg/min (<5 kg), 5–10 mL/kg/min (<30 kg), 2–5 mL/kg/min if older (≥30 kg)
- 5–10×DFR or RFFR
- ≥(5×[RFFR+PRR]/60 min)/(1−hematocrit)
(7) Anticoagulation with regular monitoring of activated clotting time
- Heparin loading 10−25 units/kg & 5−10 units/kg/hr as maintenance
- Nafamostat mesylate 0.1−1 mg/kg/hr
(8) Monitor blood pressure, body weight, coagulation, chemistry (phosphorous, potassium, magnesium, pH q 6–8 hours), and effluent volume frequently
(9) Consider removal of nutrients and medication via CRRT
AKI may cause permanent damage to the kidney by reducing the renal mass, causing renal medullary injury that is highly sensitive to ischemic injury during AKI, and following maladaptive repair and tubular atrophy [45,46]. A meta-analysis showed that adult patients with AKI have more than 8.8 fold higher risk of CKD and more than 3 fold higher risk for ESRD [46,47]. KDIGO guidelines recommend that patients with AKI should be followed up at 3 months after recovery from AKI, for renal function, blood pressure, and proteinuria [29]. As the increased risk of CKD years after AKI episodes is well known (Fig. 3) [47-52], long-term follow-up of such patients is necessary. In addition, patients should be educated to avoid exposure to nephrotoxins such as NSAIDs and radiocontrast agents, and to actively undergo treatment for diabetes, hypertension, and proteinuria, if present.
Through understanding the pathophysiology of AKI, potential therapeutic interventions, such as anti-inflammatory agents including recombinant alkaline phosphate [53], costimulatory molecule CD28 receptor antagonist [54], antioxidants, vasodilator-calcium sensitizer levosimendan [55], apoptosis inhibitors (p53 siRNA) and repair agents including bone morphogenetic protein-7 receptor agonist hepatocyte growth factor, and mesenchymal stem cells, are now under consideration [53].
AKI is common in critically ill children and is associated with a very poor outcome. As there is no proven effective pharmacological intervention for AKI, prevention and early detection are important. To prevent and recover from AKI, optimization of volume status and circulation, avoidance of nephrotoxic agents, and sufficient nutritional support are necessary. CRRT continues to be increasingly performed for treating severe AKI. Novel biomarkers and potential therapeutic interventions for AKI are being investigated.
Acknowledgments
This study was supported from the Seoul National University Hospital Research Fund (grant number: 2520160020).
Fig. 1.
Kaplan-Meier graph for hospital survival, stratified by Kidney Disease: Improving Global Outcomes stages of acute kidney injury. Reprinted from Rewa and Bagshaw. Nat Rev Nephrol 2014;10:193-207, with permission of Springer Nature[1].
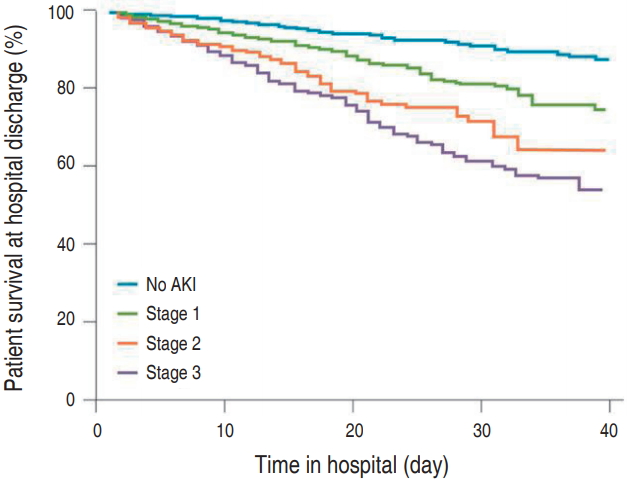
Fig. 2.
Clinical course in a patient with acute kidney injury caused by Yersinia pseudo-tuberculosis infection[14]. WBC, white blood cell; ESR, erythrocyte sedimentation rate.
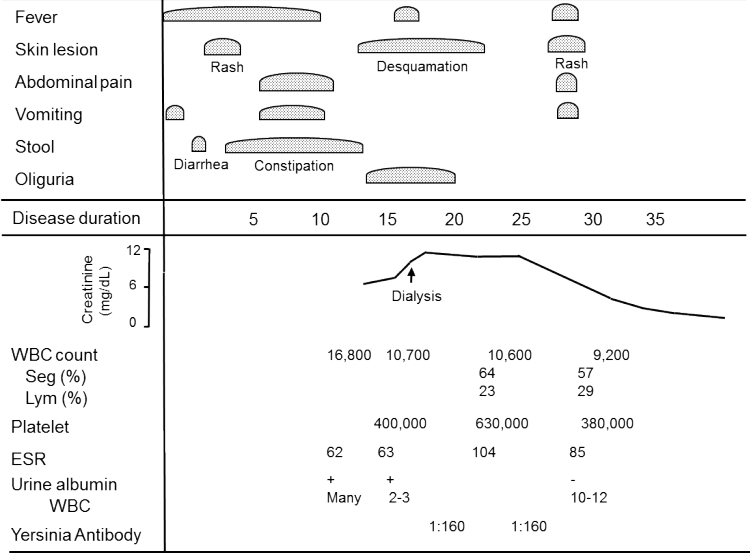
Fig. 3.
Time to end-stage renal disease in patients undergoing cardiac surgery classified according to severity of acute kidney injury[52]. AKIN, acute kidney injury network.
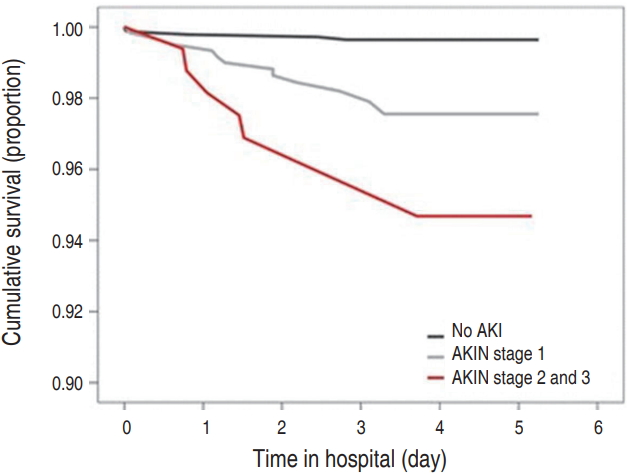
Table 1.
Table 2.
Common causes of acute kidney injury
Table 3.
Exposures and susceptibilities for nonspecific acute kidney injury [2]
Table 4.
Recommended vascular access size, available in Korea in 2018
| Body weight | Access |
|---|---|
| Newborn to 9 kg | 6.5–7F dual lumen (Gambro) |
| 9–30 kg | 8–9F dual lumen (Covidien/Gambro/MedComp) |
| >30 kg | 10–12F dual lumen (Covidien/Gambro/MedComp) |
Table 5.
Choice of filter/membrane for continuous renal replacement therapy, available in Korea in 2018
Table 6.
Choice of continuous renal replacement therapy fluid, available in Korea in 2018
References
1. Rewa O, Bagshaw SM. Acute kidney injury-epidemiology, outcomes and economics. Nat Rev Nephrol 2014;10:193–207.



2. Kellum JA, Lameire N, KDIGO AKI Guideline Work Group. Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1). Crit Care 2013;17:204



3. Selewski DT, Charlton JR, Jetton JG, Guillet R, Mhanna MJ, Askenazi DJ, et al. Neonatal Acute Kidney Injury. Pediatrics 2015;136:e463. –73.


4. Lagos-Arevalo P, Palijan A, Vertullo L, Devarajan P, Bennett MR, Sabbisetti V, et al. Cystatin C in acute kidney injury diagnosis: early biomarker or alternative to serum creatinine? Pediatr Nephrol 2015;30:665–76.


5. Nehus EJ, Laskin BL, Kathman TI, Bissler JJ. Performance of cystatin C-based equations in a pediatric cohort at high risk of kidney injury. Pediatr Nephrol 2013;28:453–61.


6. Moore PK, Hsu RK, Liu KD. Management of acute kidney injury: core curriculum 2018. Am J Kidney Dis 2018;72:136–48.


7. Kashani K, Al-Khafaji A, Ardiles T, Artigas A, Bagshaw SM, Bell M, et al. Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit Care 2013;17:R25



8. Endre ZH, Pickering JW. Acute kidney injury: cell cycle arrest biomarkers win race for AKI diagnosis. Nat Rev Nephrol 2014;10:683–5.



9. Charlton JR, Portilla D, Okusa MD. A basic science view of acute kidney injury biomarkers. Nephrol Dial Transplant 2014;29:1301–11.




10. Chawla LS, Goldstein SL, Kellum JA, Ronco C, et al. Renal angina: concept and development of pretest probability assessment in acute kidney injury. Crit Care 2015;19:93



12. Kliegman RM, Stanton BF, St. Geme JW III, Schor NF, Behrman RE, editors. Nelson textbook of pediatrics. 20th ed. Philadelphia (PA): Elsevier, 2016.
13. Taal MW, Chertow GM, Marsden PA, Skorecki K, Yu A, Brenner BM. Brenner & Rector’s the kidney. 9th ed. Philadelphia (PA): Elsevier, 2012.
14. Lee HJ, Chung HI, Choi Y, Shin MJ, Moon HR. Epidemic of acute renal failure and Kawasaki disease-like illness caused by Yersinia pseudotuberculosis Infection. J Korean Med Assoc 1988;31:747–56.
15. Grams ME, Astor BC, Bash LD, Matsushita K, Wang Y, Coresh J. Albuminuria and estimated glomerular filtration rate independently associate with acute kidney injury. J Am Soc Nephrol 2010;21:1757–64.



16. Basu RK, Zappitelli M, Brunner L, Wang Y, Wong HR, Chawla LS, et al. Derivation and validation of the renal angina index to improve the prediction of acute kidney injury in critically ill children. Kidney Int 2014;85:659–67.


17. Morgan CJ, Zappitelli M, Robertson CM, Alton GY, Sauve RS, Joffe AR, et al. Risk factors for and outcomes of acute kidney injury in neonates undergoing complex cardiac surgery. J Pediatr 2013;162:120–7. e1.


18. Sethi SK, Kumar M, Sharma R, Bazaz S, Kher V. Acute kidney injury in children after cardiopulmonary bypass: risk factors and outcome. Indian Pediatr 2015;52:223–6.


19. Prowle JR, Kirwan CJ, Bellomo R. Fluid management for the prevention and attenuation of acute kidney injury. Nat Rev Nephrol 2014;10:37–47.



20. Grams ME, Estrella MM, Coresh J, Brower RG, Liu KD. National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome Network. Fluid balance, diuretic use, and mortality in acute kidney injury. Clin J Am Soc Nephrol 2011;6:966–73.



21. Sutherland SM, Zappitelli M, Alexander SR, Chua AN, Brophy PD, Bunchman TE, et al. Fluid overload and mortality in children receiving continuous RRT: the prospective pediatric continuous renal replacement therapy registry. Am J Kidney Dis 2010;55:316–25.


22. Mah KE, Hao S, Sutherland SM, Kwiatkowski DM, Axelrod DM, Almond CS, et al. Fluid overload independent of acute kidney injury predicts poor outcomes in neonates following congenital heart surgery. Pediatr Nephrol 2018;33:511–20.



23. Bagshaw SM, Cruz DN. Fluid overload as a biomarker of heart failure and acute kidney injury. Contrib Nephrol 2010;164:54–68.


24. Lee ST, Cho H. Fluid overload and outcomes in neonates receiving continuous renal replacement therapy. Pediatr Nephrol 2016;31:2145–52.



25. Mutter TC, Ruth CA, Dart AB. Hydroxyethyl starch (HES) versus other fluid therapies: effects on kidney function. Cochrane Database Syst Rev 2013;(7):CD007594

26. Chowdhury AH, Cox EF, Francis ST, Lobo DN. A randomized, controlled, double-blind crossover study on the effects of 2-L infusions of 0.9% saline and plasma-lyte® 148 on renal blood flow velocity and renal cortical tissue perfusion in healthy volunteers. Ann Surg 2012;256:18–24.


27. Self WH, Semler MW, Wanderer JP, Wang L, Byrne DW, Collins SP, et al. Balanced crystalloids versus saline in noncritically Ill adults. N Engl J Med 2018;378:819–28.



28. Friederich A, Martin N, Swanson MB, Faine BA, Mohr NM. Normal saline solution and actated Ringer's solution have a similar effect on quality of recovery: a randomized controlled trial. Ann Emerg Med 2018;Aug 23 [Epub]. pii: S0196-0644(18)30627-9. https://doi.org/10.1016/j.annemergmed.2018.07.007.

31. Weisbord SD, Gallagher M, Jneid H, Garcia S, Cass A, Thwin SS, et al. Outcomes after angiography with sodium bicarbonate and acetylcysteine. N Engl J Med 2018;378:603–14.


32. Brunkhorst FM, Engel C, Bloos F, Meier-Hellmann A, Ragaller M, Weiler N, et al. Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med 2008;358:125–39.


33. Ho KM, Power BM. Benefits and risks of furosemide in acute kidney injury. Anaesthesia 2010;65:283–93.


34. Bellomo R, Chapman M, Finfer S, Hickling K, Myburgh J. Low-dose dopamine in patients with early renal dysfunction: a placebo-controlled randomised trial. Australian and New Zealand Intensive Care Society (ANZICS) Clinical Trials Group. Lancet 2000;356:2139–43.


35. Toma A, Stone A, Green RS, Gray S. Steroids for patients in septic shock: the results of the CORTICUS trial. CJEM 2011;13:273–6.


36. Bove T, Zangrillo A, Guarracino F, Alvaro G, Persi B, Maglioni E, et al. Effect of fenoldopam on use of renal replacement therapy among patients with acute kidney injury after cardiac surgery: a randomized clinical trial. JAMA 2014;312:2244–53.


37. Mei M, Zhao HW, Pan QG, Pu YM, Tang MZ, Shen BB. Efficacy of Nacetylcysteine in preventing acute kidney injury after cardiac surgery: a meta-analysis study. J Invest Surg 2018;31:14–23.


38. ACT Investigators. Acetylcysteine for prevention of renal outcomes in patients undergoing coronary and peripheral vascular angiography: main results from the randomized Acetylcysteine for Contrastinduced nephropathy Trial (ACT). Circulation 2011;124:1250–9.


39. Zarbock A, Kellum JA, Schmidt C, Van Aken H, Wempe C, Pavenstädt H, et al. Effect of early vs delayed initiation of renal replacement therapy on mortality in critically Ill patients with acute kidney injury: The ELAIN randomized clinical trial. JAMA 2016;315:2190–9.


40. Gaudry S, Hajage D, Schortgen F, Martin-Lefevre L, Tubach F, Pons B, et al. Comparison of two strategies for initiating renal replacement therapy in the intensive care unit: study protocol for a randomized controlled trial (AKIKI). Trials 2015;16:170




41. Macedo E, Mehta RL. Continuous dialysis therapies: core curriculum 2016. Am J Kidney Dis 2016;68:645–57.


42. Ronco C, Bellomo R, Homel P, Brendolan A, Dan M, Piccinni P, et al. Effects of different doses in continuous veno-venous haemofiltration on outcomes of acute renal failure: a prospective randomised trial. Lancet 2000;356:26–30.


43. VA/NIH Acute Renal Failure Trial Network; Palevsky PM, Zhang JH, O'Connor TZ, Chertow GM, Crowley ST, et al. Intensity of renal support in critically ill patients with acute kidney injury. N Engl J Med 2008;359:7–20.



44. Uchino S, Bellomo R, Morimatsu H, Morgera S, Schetz M, Tan I, et al. Discontinuation of continuous renal replacement therapy: a post hoc analysis of a prospective multicenter observational study. Crit Care Med 2009;37:2576–82.


45. Venkatachalam MA, Weinberg JM, Kriz W, Bidani AK. Failed tubule recovery, AKI-CKD transition, and kidney disease progression. J Am Soc Nephrol 2015;26:1765–76.



46. Sigurjonsdottir VK, Chaturvedi S, Mammen C, Sutherland SM. Pediatric acute kidney injury and the subsequent risk for chronic kidney disease: is there cause for alarm? Pediatr Nephrol 2018;33:2047–55.



47. Coca SG, Singanamala S, Parikh CR. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int 2012;81:442–8.


48. Wu VC, Huang TM, Lai CF, Shiao CC, Lin YF, Chu TS, et al. Acute-onchronic kidney injury at hospital discharge is associated with longterm dialysis and mortality. Kidney Int 2011;80:1222–30.


49. Chawla LS, Eggers PW, Star RA, Kimmel PL. Acute kidney injury and chronic kidney disease as interconnected syndromes. N Engl J Med 2014;371:58–66.



50. Mammen C, Al Abbas A, Skippen P, Nadel H, Levine D, Collet JP, et al. Long-term risk of CKD in children surviving episodes of acute kidney injury in the intensive care unit: a prospective cohort study. Am J Kidney Dis 2012;59:523–30.


51. Askenazi DJ, Feig DI, Graham NM, Hui-Stickle S, Goldstein SL. 3-5 year longitudinal follow-up of pediatric patients after acute renal failure. Kidney Int 2006;69:184–9.


52. Chew ST, Ng RR, Liu W, Chow KY, Ti LK. Acute kidney injury increases the risk of end-stage renal disease after cardiac surgery in an Asian population: a prospective cohort study. BMC Nephrol 2017;18:60




53. Benoit SW, Devarajan P. Acute kidney injury: emerging pharmacotherapies in current clinical trials. Pediatr Nephrol 2018;33:779–87.








 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link PubMed
PubMed Download Citation
Download Citation


