Discussion
Ret-He is a flow cytometry technique and one of the latest hematology tools to estimate Hb content in reticulocytes in order to describe the actual condition of erythropoiesis and to detect early IDA. Several previous studies have shown the role of Ret-He as a diagnostic tool for IDA. Rungnggu et al. [
4], in a study of Ret-He for diagnosis of IDA, suggested that low Ret-He levels were significantly associated with IDA in children (
P=0.005), with a cutoff point of Ret-He 27.8 pg/L, sensitivity of 43.8%, specificity of 85.3%, positive predictive value (PPV) of 58.3% and negative predictive value (NPV) of 76.3% with odds ratio 4.5 (95% CI, 1.1–17.7), and it was said that Ret-He has high enough specificity that it can be used as a tool for early detection screening of children with IDA. Toki et al. [
5], in a study of Ret-He as a potential marker for diagnosis of iron deficiency, showed that there was a correlation between decreased Ret-He and iron deficiency (
P=0.016). The studies above used Ret-He to diagnose IDA with the cutoff of <27.8 pg/L. Esther published her thesis on the role of Ret-He in iron deficiency screening in children aged 6 months to 5 years. Of the 94 children who were examined for Ret-He levels with ferritin as a gold standard, Ret-He results were obtained 87 samples a cutoff of 28 pg/L, 81.3% sensitivity, 93.6% specificity, 72% PPV, 96% NPV, 12.7 LR (+), and 0.2 LR (-). Sanyoto presented results from 87 children with IDA which showed that Ret-He can play a role in diagnosis and is an indicator of therapeutic response to iron preparations with 97.2% sensitivity, 66.67% specificity, 67.30% PPV, and 97.14% NPV. It can be argued that Ret-He levels in the earliest cases of IDA increased when considering Hb, reticulocyte, and erythrocyte index. In addition, Ret-He is also useful for assessing therapeutic response in children with IDA.
The most important function of iron in the body is the development of the nervous system required in myelinization, neurotransmitters, dendritogenesis, and also neural metabolism [
6]. Iron deficiency dramatically affects the cognitive function, behavior, and growth of infants. Iron is also a source of energy for the muscles that affect physical endurance and ability to work primarily in adolescents. In healthy adolescents with no accompanying infectious disease, IDA is most often caused by low iron intake [
7]. Children with IDA can be given oral elemental therapy with a dose of 3-mg/kg body weight before meals or 5-mg/kg body weight after meals divided into 3 doses. Iron deficiency resulting from inflammatory or infectious processes can be treated with 3–6 mg/kg body weight per day of iron therapy for 3–6 weeks [
8]. In this study, the SF iron preparation is from a government program and is usually used to treat cases of anemia in children and pregnant women. This SF is obtained from the provincial health office. One 300-mg tablet of SF is equivalent to 60 mg of elemental iron. So, because the average weight of the children sampled was 25.603–26.719 kg, they were given a dose of 2×1 SF sugar-coated tablet each day for 14 days.
L. reuteri is part of the gastrointestinal microflora in infants and children.
L. reuteri ferments lactose carbohydrates or undigested oligosaccharides in the large intestine to produce an acidic atmosphere that improves bowel motility by improving bowel peristalsis movement, which finally helps absorption and aids in fecal outbreaks.
Reuteri is suspected to have an anti-
Helicobacter pylori effect [
9]. For the addition of intestinal microbiota, in this study, we used a single strain probiotic
L. reuteri DSM 17938 given with a dose of 3×10
8 CFU daily for 14 days.
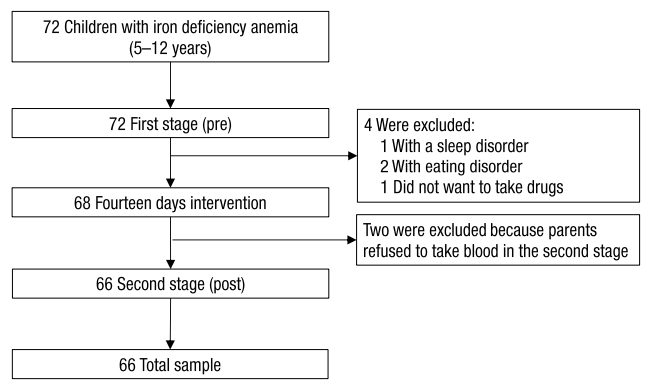
In this study, Ret-He examination was done in elementary school children aged 5–12 years, who were found to be pale on physical examination and had a PCV value ≤36%. The selected school is a school which, according to mapping data from the local primary healthcare provider, has a high rate of anemia. Before the first stage of the examination, a complete history was made for all the subjects, and complete data were gathered along with active phone numbers. The researcher also explained to the parents the planned drug administration and blood test that would be done after 14 days of intervention. Subsequently all the parents signed an informed consent form. After that, the results of examination for a Ret-He <27.8 pg/L were evaluated by treatment intervention with subject divided into 2 groups: group 1 was given an iron preparation as well as L. reuteri DSM 17938 and group 2 was given only the iron preparation. Drugs were submitted directly to the patients’ parents, along with education on how to use the drugs and what procedures would be done. Subjects do not need to add iron or another probiotic intake from milk or other processed products containing probiotics for 14 days. Monitoring of medication adherence and side effects of the drug followed. This was done through personal contact and one supervisor, who also brought the medication from primary healthcare provider, checking subjects’ medication use once every three days. On the 15th day, the Hb, Ht, and Ret-He levels of the subjects in both groups were examined.
This study supports the theory and previous studies that say that probiotics have a role in iron absorption. Probiotics work directly on epithelial cells to increase the expression and excretion of mucin from goblet cells as well as β defensin, which inhibits proliferation of pathogenic or commensal germs and affects the integrity of the mucosal barrier. Probiotics also improve tight junction stability, which reduces the permeability of the pathogens and their products. So, intestinal mucosa does not bind to the pathogen but more to the substances that the body needs, such as iron. Probiotics affect mucosal immunity by increasing the number of sIgA-producing cells in the lamina propria, which prevents colonization on epitel by binding to bacteria and antigens so that it affects gastrointestinal balance.
L. reuteri improves the apical surface of the intestine that affects divalent metal transporter-1 (DMT-1), which plays a vital role in the absorption of the iron preparation used [
10]. Administering probiotics together with iron preparations is expected to provide better outcomes for patients with IDA, provided that the probiotics are administered earlier than iron preparations.
A previous study said that an iron-fortified fermented milk beverage that was added with probiotics increased the iron status of preschoolers after 35 days of consumption. The compound used in fortification is an amino acid clamp iron, which according to the literature dissolves in the intestine and is easily absorbed. In addition,
Lactobacillus may help balance intestinal microflora and improve nutritional availability [
11].
Paracellular permeability is determined by the structure of the complex of junctions that are localized close to the epithelial cells, where the body gets the input of iron from food in the gut. The iron absorption process occurs mostly in the duodenum and proximal jejunum. The apical membrane of enterocytes in the duodenum acts as a transporter for iron from the intestine lumen to the enterocyte. The most important transporter molecule is DMT-1, with
L. reuteri’s role on the surface of the intestine and effect on the intestinal paracellular permeability affecting DMT-1 in iron absorption [
12].
Sazawal et al. [
13] conducted a study on the effects of
Bifidobacterium lactis HN019 and milk containing oligosaccharide prebiotic on iron status, anemia, and growth in children aged 1–4 years. Results of this study were that consumption of milk with added probiotics and prebiotics within 1 year reduced the risk of anemia and iron deficiency by 45% (95% CI, 115; 66%;
P=0.01) and caused weight gain of 0.13 kg/yr (95% CI, 0.03–0.23;
P=0.02). The study concluded that preschool children that were given probiotic-enriched fermented milk showed a slight decrease in iron deficiency and an increase in body weight.
Emara et al. [
14] from Egypt conducted a double-blind clinical trial to find out whether the addition of
L. reuteri probiotics to the standard triple therapy would improve the eradication of
Helicobacter pylori. In conclusion, the addition of
L. reuteri probiotics can improve the Gastrointestinal Symptom Rating Scale score of dyspeptic patients receiving triple therapy.
Silva et al. [
11], in previous experiments in 2002, showed a significant increase in ferritin levels observed in 25% of preschool children after a 35-day intervention with probiotic microorganisms. Siva et al. [
11], in subsequent trials in 2008, evaluated the effects of fermented milk which was fortified with iron chelation with and without probiotic bacteria (
Lactobacillus acidophilus) on growth and iron status of preschoolers in long-term intervention. The result was a positive correlation (
P=0,02) between iron intake and Hb in the probiotic group. Results also showed a higher increase (38.2%, n=45) in the Hb level compared to the group given drink without probiotic microorganisms (control) (30.2%, n=84). Hematologic analysis performed in this study was based on the hypothesis that probiotics would increase the bioavailability and storage of iron in the body. The results of this study showed a significant increase in mean corpuscular hemoglobin concentration (MCHC) found in groups given probiotic beverages, indicating higher red blood cell saturation and reduced hypochromia in children [
11]. Oda et al. [
15] in their study also showed that
L. acidophilus increased the bioavailability of fermented iron in an animal experiment.
The study conducted by Zimmermann et al. [
16] showed that iron fortification in children causes the gut microbiota profile to be more pathogenic due to the increased in pathogenic enterobacteria and decrease in
Lactobacilli, and may increase the frequency of diarrhea. Iron supplementation can lead to the formation of free radicals, which have a negative impact on child endurance. In addition, at the time of getting iron supplementation, there is an imbalance of intestinal microbiota due to increase in intestinal bacteria pathogens, which in turn increases the incidence of diarrhea. It has been explained that supplementation and fortification is the main effort in tackling the anemia problem, but, on the other hand, may also have the negative impact of microbiota imbalance in the gastrointestinal tract, which can cause diarrhea. Therefore, we need a way to overcome this using probiotics to maintain the number of
Lactobacilli and
bifidobacteria in the intestine during the administration of iron. This step is expected to maintain the balance of intestinal microbiota and suppress the increase of enteropathogenic bacteria so that the program of supplementation and iron fortification can still be carried out without worrying about its negative effects. This study describes the role of intestinal microbiota in the absorption of substances that the body needs by the intestine, such as the absorption of iron. The failure of IDA therapy that has occurred so far may be related to intestinal microbiota balance. Administering probiotics to children with IDA is expected to provide good outcomes. In cases of IDA where probiotics are not available, it is advisable to consume food products that contain probiotics [
17].
Hoppe et al. [
18] had a pre-post study in women of reproductive age using probiotic strain
Lactobacillus pantarum 299v with serum iron (SI) as a study indicator. The 21 subjects were divided into 2 groups, 10 in the control group and 11 in the case group, and the results obtained show that there was a significant increase in iron absorption in the case group, which was given a drink containing
L. pantarum 299v, compared with the control group with
P=0.08.
Most studies of probiotics associated with iron absorption concluded that there was a correlation between probiotics and iron absorption, but there were also previous studies whose results differ from this study. Agustina et al. [
19] conducted a study in Indonesia in 2013 using milk intervention with probiotics
L. reuteri and
Lactobacillus casei in children aged 1–6 years. The results concluded that milk intervention with the probiotics
L. reuteri significantly increased body weight, but not iron and zinc status. Asemi et al. [
20] studied the effect of daily consumption of yogurt probiotics on levels of calcium, iron, and liver enzymes in pregnant women. The results of this study suggest that consumption of probiotic yogurt retains serum calcium levels compared with those not consuming yogurt (
P=0.01), this significant difference is shown by a significant decrease in serum calcium levels (-1.7 mg/dL,
P<0.0001). But there was no significant difference between the groups of pregnant women who consumed yogurt and those who did not consume yogurt in serum iron and liver enzyme levels. This may be related to confounding variables of the daily consumption patterns of the study subjects.
This study has limitations. First, researchers cannot exclude all confounding variables that may affect results after the intervention. For example, some children’s diets that may not have been accurately reported by parents. Also, 2 blood tests were done during 14 days cause some samples dropped. Based on the results of this study, it can be concluded that intervention with iron preparations and L. reuteri DSM 17938 in children with IDA for 14 days leads to a higher increase in levels of Ret-He than does intervention with iron preparations only. L. reuteri DSM 17938 has a beneficial role in the absorption of iron from the intestinal mucosa.






 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link PubMed
PubMed Download Citation
Download Citation

