Introduction
Menarche, defined as a young womanŌĆÖs first menstrual period, is a landmark of pubertal development in females. Age at menarche (AAM) is a key maturity indicator of a female's healthy transition from childhood into young adulthood and an important clinical indicator of her physical, nutritional, and reproductive health [1]. AAM is influenced by several factors, including genetics, ethnicity, geography, socioeconomic status, and nutritional status [2-4].
AAM has decreased continuously and rapidly in Asian countries, including Korea, probably because of improvements in nutrition [5]. In Korea, AAM has declined from 15ŌĆō17 years in the 1960s to around 14 years in the 1980s and 12.6 years in the 1990s [5]. Thus, the proportion of females with early menarche, commonly defined as an AAM <12 years old, has also increased in Korea [6].
Female adolescents who experience early puberty and early menarche are known to be at a greater risk of psychosocial problems such as delinquency and risky sexual behavior [7]. Furthermore, they achieve a shorter final height and develop obesity and several physical health problems such as menstrual problems, compared to adolescents who experience menarche at a later age [8,9]. Furthermore, early menarche is a well-known risk factor of chronic cardiometabolic disease and related early mortality in adulthood [10]. Population-based Korean studies also showed an association between early AAM (<12 years) and the risk of obesity, insulin resistance (IR), metabolic syndrome (MetS), nonalcoholic fatty liver disease (NAFLD), diabetes, breast cancer, and cardiovascular disease (CVD) in adulthood [10-13].
In pediatrics, establishing risk factors is important in disease prevention in later life. An early AAM can be a good marker for predicting future metabolic problems. Therefore, this review aimed to: (1) review the secular trend of AAM and early menarche in Korean females; (2) review the factors and conditions related to AAM; (3) find proof of whether early menarche is a risk factor for obesity, IR, MetS, NAFLD, diabetes, and cancer in adult Korean females; and (4) address how to prevent early menarche and discuss its consequences by reviewing its proposed mechanism. To this end, we reviewed the literature, particularly meta-analyses and prospective studies, regarding menarche in Korean female. We hope this review will help pediatricians comprehensively understand the lifetime health problems of women due to early menarche and in preventing early menarche and its complications.
Secular trends in pubertal timing and AAM
Breast budding is the herald of puberty in girls. The age at the onset of breast development seems to have declined markedly over the last two decades [14]. Before the 1980s, the mean age at the onset of breast development was approximately 11 years in American and European countries [15]. However, the mean age at puberty onset in girls was <10 years from the 3rd National Health and Nutrition Examination Survey (NHANES III), 1988ŌĆō1994 [16].
A gradual decline in AAM was reported over the past few decades in Western countries [17]. In Europe, the AAM decreased from approximately 17 years in the early 19th century to approximately 13 years by the mid-20th century and stayed relatively stable with a variation of 0.5 years depending on study population since the 1960s [18]. However, this secular trend is rapid and ongoing in Asian countries, including Korea. In China, the AAM has fallen 0.7 years per decade over a 40-year period; most recently, it was 12.76 years [19].
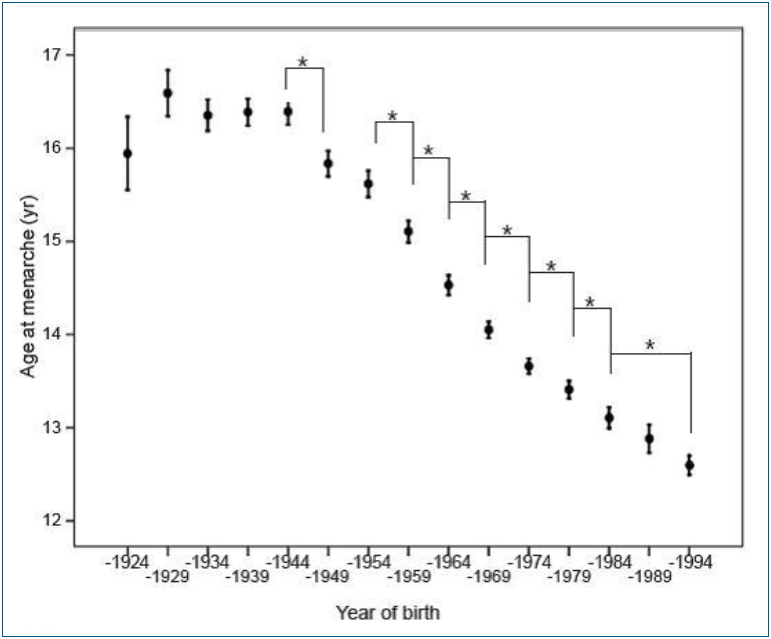
In Korea, the mean AAM for females born in 1925ŌĆō1929 was 16.59┬▒1.82 years. The AAM decreased to 13.11┬▒1.52 years for those born in 1980ŌĆō1984 and to 12.60┬▒1.14 years for those born in 1990ŌĆō1994 (Fig. 1) [5]. Young Korean women aged 18ŌĆō30 years now have a mean AAM of 12.7┬▒1.6 years, which is 1.5 years earlier than that of their mothers. Furthermore, the prevalence of early menarche (<12 years) is 22.3%, while the percentage of subjectsŌĆÖ mothers with early menarche was only 2.5% [8]. This trend might be due to changes in the socioeconomic environment, possibly acting through changes in nutrition and exercise patterns. South Koreans rapidly accepted Western culture, especially the Western diet, after the 1988 Olympic Games. On the contrary, North Korean refugees still showed an AAM around 16.0┬▒2.1 years [20].
Determinants and risk factors of early menarche
The AAM can be determined by genetic and environmental factors and their interactions. However, the observed secular trend in AAM in this ethnicity may suggest that environmental rather than genetic factors are the major contributors to increasing rates of early menarche. The factors and conditions related to early menarche are listed in Table 1.
1. Genetic determinants of menarche
Twin and familial studies of AAM indicated a 57%ŌĆō82% of variance in the timing of puberty could be explained by heritable factors [21,22]. Racial differences in pubertal maturation might reflect genetic factors.
Genome-wide association studies on AAM performed in Caucasian females confirmed associations at two loci: 6q21 (in or near the LIN28B gene) and 9q31.2 [23,24]. Effect sizes were estimated at only 0.12 years earlier per effect allele for LIN28B and 9q31.2. Menarche signals were enriched in imprinted regions, with three loci (DLK1-WDR25, MKRN3-MAGEL2, and KCNK9) [25].
Recently, a womanŌĆÖs AAM has been suggested as a likely contributor to earlier pubertal development and early menarche in her female offspring [26]. In our study of Korean mother-daughter pairs, the correlation between the AAM of mothers and their daughters was 0.245, comparable to figures of 0.23ŌĆō0.39 in other countries [27,28]. Furthermore, the daughters of mothers with an earlier AAM (<13 years) were more likely to experience early menarche (<12 years) themselves (earlier, 35.6%; reference, 25.8%; P<0.05). The odds ratio (OR) for early menarche of such daughters compared with reference was 1.56 after the adjustment for confounders (under review).
2. Nongenetic determinants of menarche
Despite the major role of genetic factors in AAM, environmental factors have gained increasing attention due to their potential for control. It is well-known that AAM is related to obesity, nutrition (especially fructose), endocrine-disruptor channels (EDCs), and physical activity. Furthermore, small for gestational age (SGA) and precocious puberty are risk factors for early menarche.
1) Obesity (body fat)
The ŌĆ£critical weight hypothesisŌĆØ suggested by Frisch-Revelle states that the onset of menarche is associated with increased body fat, 22% or 48 kg of body weight, in pubertal girls [29]. Leptin conveys information about fat mass and distribution to the hypothalamus during puberty [30]. It stimulates the pul satile release of gonadotropin-releasing hormone (GnRH) in the hypothalamus, which serves as a signal for the onset of menarche [31]. Most studies showed the association between high-fat content in infancy (or prepuberty or puberty) and earlier AAM. A higher subcutaneous fat levels and a high prepubertal body mass index (BMI) are associated with an increased likelihood of early (<11 years) menarche [3].
2) Nutritional habits
An increased energy intake was associated with early menarche. Food quality also influences puberty. Formula feeding during early infancy might promote weight gain and earlier AAM in Asians and Caucasians [32,33], whereas breastfeeding may decrease the risk of early puberty. However, recent studies found no association between milk consumption, breastfeeding duration (more than 3 months), and pubertal timing in Chinese children [34]. Berkey et al. [35] demonstrated that a high animal versus vegetable protein ratio at the ages of 3ŌĆō5 years is associated with early menarche after controlling for BMI. Dietary fiber might prevent early AAM through its effect on estrogen metabolism [36]. Dietary fiber might reduce estrogen reabsorption and deconjugation in the gut.
3) Sugar-sweetened beverages
Sugar-sweetened beverages (SSBs) include sodas and soft drinks as well as other beverages with added sugars such as fruit-flavored drinks, sports and energy drinks, and sweetened coffees and teas. SSBs comprise approximately 30% of the fructose consumed in the US diet [37]. SSBs caused early AAM in a prospective study of US girls [38]. Girls consuming >1.5 SSBs per day had an estimated 2.7-month younger AAM. The National Heart, Lung, and Blood Institute Growth and Health Study recruited 2,379 girls aged 9ŌĆō10 years and followed them for 10 years. The incidence of early menarche (menarche age <11 years) occurred in 8.3% of the girls. After the adjustment for confounders and premenarcheal percentage body fat, a greater consumption of SSBs was associated with a higher risk of early menarche [39]. The relative risk (RR) for one serving/day was 1.47.
4) Exercise
Some physical activities, in combination with a nutritional deficiency, may influence the onset of puberty [40] and lead to a mean 1-year delay in AAM in Caucasians [41]. AAM was positively associated with physical activity (at least 2 hours daily) in a group of Colombian university women [42]. Menarche tends to occur later in athletes, including ballet dancers, then in the general population, but not in swimmers, suggesting that intense exercise delays puberty [43]. The most probable explanation for the lack of a pubertal delay in swimmers is that the normal body fat composition of swimmers balances the negative hypothalamic effect on GnRH pulsatile exerted by intensive exercise.
5) Endocrine-disruptor chemicals
EDCs are a group of hormonally active agents. People are directly or indirectly exposed to EDCs every day because EDCs are widely used in the industry for almost a century [44]. EDCs are used as industrial solvents/lubricants (polychlorinated biphenyls [PCBs], polybrominated biphenyls, dioxins), plastics (bisphenol A [BPA]), plasticizers (phthalates), and pesticides (dichlorodiph enyltrichloroethane) [45]. Phthalates and PCBs are associated with earlier breast development and menarche, respectively [46]. BPA is well-known for its molecular estrogen-like actions and showed a close relationship between urinary BPA, body weight, and early puberty [47]. Low doses of lead may be associated with the earlier onset of menses, whereas high-dose exposure may delay AAM [41]. Some personal care chemicals, such as hair care products, used every day during childhood, can also lower AAM [48].
6) SGA and rapid early weight gain
Girls born SGA showed early pubertal onset and menarche by about 5ŌĆō10 months [49]. A lower postnatal weight gain has a stronger association with earlier AAM in females with intrauterine growth retardation. A rapid early infancy weight gain from birth to age 2 months as well as 2ŌĆō9 months predicted subsequent greater adiposity and earlier AAM in girls [32].
7) Precocious puberty
Precocious puberty is defined as the development of secondary sexual characteristics before 8 years of age in girls. Premature activation of the hypothalamicŌĆōpituitaryŌĆōgonadal axis in central precocious puberty stimulates the production of sex steroids that stimulate linear growth and accelerate bone age advancement and result in early menarche. In untreated children with central precocious puberty, early growth acceleration and premature fusion of the epiphyseal growth plates result in an impaired final height [50,51].
Early menarche and its adverse consequences in later life
Several studies have established an association between early menarche and the risk of cardiometabolic diseases in adulthood. The adverse consequences in later life (including late adolescents, young adults, and adults) associated with early menarche are listed in Table 2.
1. Short adult height
Early menarche increased the risk of adult short stature in young Korean women. According to the European Prospective Investigation into Cancer and Nutrition study, based on 286,205 women, their final height was 0.35 cm (range by country, 0.13 to 0.50 cm) shorter when menarche occurred 1 year earlier [52]. Furthermore, a 1-year decrease in AAM decreased the standing height and leg length by 0.76 and 0.41 cm, respectively, in the USA birth cohort [53].
In Korea, we found that 1-year decrease in the AAM was associated with a 0.445 cm final height loss in young Korean women [8]. We also found that young women with early menarche had a 10.5% chance of having a short adult stature (Ōēż153 cm), which was 2.62-fold higher than those with later menarche. These findings are due to the earlier closure of epiphyseal growth plates due to the increase in ovarian estrogens [54,55]. A high dose of estrogen binding to its receptors in the growth plate cartilage might cause early epiphyseal fusion by advancing growth plate senescence [55].
2. Obesity
Obesity in adulthood is closely related with early menarche. Several studies observed an inverse relationship between AAM and adult BMI [56-59]. Although the causal relationship between AAM and obesity remains unclear, earlier menarche and obesity are strongly associated with chronic cardiometabolic diseases in adulthood.
Freedman et al. [57] advocated that increasing childhood obesity rates result in both early menarche and adulthood obesity. Girls with early menarche, including Koreans, reportedly have greater body fat content in childhood [20,57]. However, Pierce and Leon [58] stressed that earlier menarche in itself might cause obesity in adults rather than be a proxy marker of sexual maturation. The secular change of AAM was also found in normal-weight girls [59]. Menarche is accompanied by a rapid increase in body weight [60,61]. High plasma estradiol levels and low sex hormonebinding globulin (SHBG) levels due to puberty are associated with adiposity [62]. In our study based on Korea National Health and Nutrition Examination Survey (KNHANES), a younger AAM is associated with current BMI of adults after control for age. The OR of obesity in women with early menarche was 1.845 times higher than that in those who experienced menarche after 12 years [5].
3. Metabolic syndrome
MetS is the combination of central obesity, increased blood pressure (BP), glucose intolerance, and dyslipidemia [63]. MetS is a main target for CVD prevention, as MetS is prospectively associated with an increased risk of CVD-related morbidity and mortality in European and Asian subjects [63,64].
Early menarche is associated with MetS in most Western studies [65-67]. The Bogalusa Heart Study found that early menarche is characterized by excess body fat content and insulin in early childhood and a higher prevalence of MetS in young adulthood [65]. The KORA F4 (Cooperative Health Research in the Region of Augsburg, South Germany, 2006ŌĆō2008) also showed that early menarche is associated with MetS in women aged 32ŌĆō81 years; after the adjustment for age and additional confounders, including lifestyle and reproductive history [67], that association was partially mediated by weight gain and increased BMI after 25 years of age. In our study based on KNHANESIV data, females with early menarche had a higher prevalence of central obesity, elevated fasting glucose, and elevated BP in the 20ŌĆō30 years group. The OR for MetS was 3.54 in premenopausal Korean women after the adjustment for age and another confounder [10].
4. Insulin resistance
The role of early menarche in the development of MetS in later adulthood is uncertain. The most likely explanation is that early menarche in adolescents and MetS in adulthood are consequences of IR. Hyperinsulinemia, usually but not always accompanied by obesity, is thought to play a mediating role in the development of early puberty [68]. Hyperinsulinemia or IR independently increased the risk of MetS and coronary heart disease (CHD) risk after the adjustment for other confounders in the Helsinki Policemen Study [69]. Among Koreans, premenopausal females over 30 years of age with early menarche had a higher prevalence of central obesity and IR [10]. The OR for IR was 2.98 after the adjustment for confounders.
In pediatrics, the best example is children born SGA. In a longitudinal cohort study, SGA children had greater adiposity and IR during catch-up growth [70]. SGA girls had early puberty and a lower AAM [64]. After menarche, SGA girls also showed hyperinsulinemia, higher androgen levels, and lower SHBG levels [71].
5. Nonalcoholic fatty liver disease
NAFLD is a spectrum of disease activity that ranges from simple hepatic steatosis to nonalcoholic steatohepatitis [72]. Epidemiological studies have shown that NAFLD may precede MetS onset and its complications and is associated with an increased risk of future CVD-related morbidity and mortality [73,74].
Mueller et al. [75]. first reported that earlier menarche is positively associated with NAFLD in the CARDIA study. They suggest that a 1-year earlier AAM is associated with a 10% increased the risk of NAFLD and visceral ectopic fat deposit in middle adulthood (43ŌĆō55 years of age) after the adjustment for other confounders. In our study, the OR for NAFLD in Korean women with early menarche compared to reference was 3.04. Further adjustment of mediators, like obesity or IR, attenuated the association to 1.91 and 2.17, but the results remained significant [11].
6. Type 2 diabetes mellitus
Type 2 diabetes mellitus (T2DM) is a metabolic disorder characterized by a high blood glucose in the context of IR, a key factor in its development [76]. Prospective studies showed that IR, based on Homeostatic Model Assessment of Insulin Resistance, persists for and is a powerful independent predictor of T2DM [77]. A 2.2-time increase in fasting insulin concentration was linked to a 2-fold increase in the future incidence of T2DM over a 24-year follow-up [78].
Several studies, including ours, reported that an early menarche is associated with a higher risk of T2DM [79-82]. In the NursesŌĆÖ Health Study I & II, the RR of T2DM in the young female cohort (40ŌĆō50 years) with early menarche was 1.40 versus 1.18 in the old cohort [79]. In the Atherosclerosis Risk in Communities study, the OR for diabetes was 1.41 after adjustment for adiposity and lifestyle [80]. In our study, the OR of diabetes was 3.61-fold after the adjustment for age in Korean women aged 20ŌĆō50 years [12]. Thus, some authors insist that the history of early menarche was a more influential factor than the current BMI or adiposity [81,82].
7. Cardiovascular diseases
CVD are the number one cause of death worldwide [83]. In Korea, around 22% of deaths are attributable to CVD [84]. In the past, many studies showed an association between AAM and the risk of CVD; increasing year of AAM lowers the risk of CVD [85-87]. The UK Million Women Study and Chinese birth cohort recently reported a significant increase in CHD risk for both early and late menarche [88,89]. In the UK Million Women Study, the risk of CHD was lowest at a menarcheal age of 13 years, and the risk was highest in a woman who experienced menarche at Ōēż10 years of age with a RR of 1.27. In Korea, a 51% reduction in mortality associated with CHD was reported in women with early menarche (<17 years) in a Kangwha cohort study [90]. The prevalence of women with a Ōēź10% or Ōēź20% 10-year Framingham Coronary Heart Disease Risk Point Scale score was higher in those with early menarche (<13 years) than in other groups after the adjustment for confounding factors [91]. The results were consistent with the previous meta-analysis, which reported a 3% higher probability of death of all causes for each decreasing year of AAM [13].
8. Cancer risk
Breast cancer is a hormone-related cancer like ovarian and endometrial. In Korea, the incidence of breast cancer increased rapidly from 24.5 in 1999 to 50.7/100,000 in 2012 and 2nd most prevalent female cancer [92]. A meta-analysis of 117 epidemiological studies showed that the breast cancer incidence risk increased by 5% when menarche occurred 1 year earlier [93]. In a meta-analysis of eight prospective endometrial cancer studies, the risk of the highest AAM was 32% lower than that of the lowest AAM and showed that the cancer risk decreased by 4% when AAM was delayed by 2 years [94]. In Korea, women with early menarche (Ōēż12 years) were at increased risk of developing breast cancer with a hazard ratio of 1.57 in the Korean Heart Study [95]. Among a cohort of British women, those with early menarche (<12 years) had a 1.25-times higher cancer mortality rate and a 1.22-times higher all-cause mortality rate after the adjustment for age, BMI, and other confounders [96].
Proposed mechanism of early menarche
The exact mechanism of how early menarche contributes to the development of cardiometabolic derangement (obesity, IR, MetS, NAFLD, diabetes, breast cancer, CVD) in later adulthood is not known.
The most popular and simple explanation is that early menarche in adolescents and cardiometabolic derangement in adulthood are consequences of childhood obesity. Childhood adiposity is known to trigger puberty and, as a result, early menarche by adipocytes and related hormones [71,97]. Childhood obesity progresses to adult obesity [97]. Obesity is well-known to be closely related to IR, MetS, NAFLD, diabetes, breast cancer, and CVD [73,98,99].
The other more complex and fine explanation is that early menarche in adolescents and metabolic derangement in adulthood are consequences of IR or hyperinsulinemia. Hyperinsulinemia, which is usually but not always accompanied by obesity, is thought to play a mediating role in the development of early puberty [66]. Furthermore, hyperinsulinemia or IR, accompanied by inflammation, independently increased the risk of MetS, NAFLD, T2DM, breast cancer, and CVD [69,77]. Hyperinsulinemia reduces SHBG levels, which in turn leads to greater bioavailability of the sex steroids [100]. Decreased serum SHBG levels are associated with early puberty [101]. SHBG is also a strong predictor of MetS, IR, and independent of total fat mass during puberty and might persist into adulthood [102].
Decreasing fructose intake to prevent early menarche
The next question is what causes obesity or IR. The Westernized lifestyle is associated with an increasing prevalence of obesity worldwide. The Westernized lifestyle is characterized by sedentary behavior or low physical activity and a Western pattern diet (WPD). The WPD is a modern dietary pattern that is generally characterized by high intakes of processed meat, pre-packaged foods, fried foods, high-fat dairy products, eggs, refined grains, potatoes, corn (and high-fructose corn syrup), and high-sugar drinks [103]. In contrast, a healthy diet has higher proportions of unprocessed fruits, nuts, vegetables, whole-grain foods, poultry, and fish.
In Korea, as a result of the rapid economic growth and the adoption of a more Western lifestyle over the past four decades, the food content shifted from a traditional Korean diet (wholegrain rice, vegetables, and kimchi) to WPD (sugar, eggs, and oil) [104]. Between 1998 and 2010, the consumption of sugar, sugar with coffee, and fruit intake were significantly and continuously increased. Strangely, energy intakes were significantly decreased by approximately 13% in adults over 20 years. During that period, the prevalence of obesity (BMI Ōēź25 kg/m2) among South Korean adults also increased from 25.7% in 1998 to 37.9% in 2013 [105]. Furthermore, according to the Korea Health Statistics, the percentage of obese adolescent boys and girls has increased considerably from 17.0% and 10.2% in 2005 to 19.1% and 14.3% in 2016, respectively [106].
A recent KNHANES study reported that SSB consumption increased dramatically from 58 g/day in 2008 to 101 g/day in 2011, and beverages including soda, coffee, and fruit and vegetable drinks are the major source of sugar intake from processed foods in the Korean population [107]. For Korean adolescents, carbonated beverages is the number one source of sugar and the consumption of SSBs is on the rise [107,108]. For Korean children aged 6ŌĆō11 years, the mean daily total sugar intake was 79.31 g/day (boys, 80.00 g/day; girls, 78.57 g/day). Children with a beverage intake Ōēź200 mL/day had a higher total sugar intake (107.97┬▒3.24 g/day vs. 69.13┬▒1.52 g/day) [109]. Furthermore, 47.9% of children consuming more than 10% of their energy intake from total sugar in processed foods. It must be corrected considering the World Health Organization recommendation that the intake of free sugars be less than 10% of oneŌĆÖs total energy intake due to its deleterious health effects [110]. The excessive consumption of fructose and sucrose is associated with deleterious effects on the metabolism and an increased risk of visceral adiposity, MetS, NAFLD, IR, and cardiometabolic risk [111-115]. No study has directly assessed the association between dietary components and early menarche. However, a meta-analysis suggested that early menarche and insulin resistance are significantly associated [116]; thus, it can be inferred that dietary components and early menarche are indirectly related.
Positive associations were also observed between SSB intake and obesity, MetS, NAFLD, insulin resistance, and cardiometabolic risk in Korean women. The OR for obesity in Korean children with a beverage intake of Ōēź200 mL/day significantly increased by 1.83 times versus that of children with a beverage intake of <200 mL/day after the adjustment for confounding variables [109]. Korean women who consumed Ōēź4 servings/week of SSBs were at a significantly higher risk of MetS than infrequent consumers [117]. Another Korean cohort study reported that subjects in the highest SSB consumption group were at a significantly greater risk (21%) of developing hypertension [118].
We previously postulated that an excessive consumption of fructose might underlie the development of NAFLD, IR, and MetS [119]. Fructose is consumed either as sucrose (50% fructose) or as high-fructose corn syrup (42% or 55% fructose). Although both glucose and fructose are categorized as carbohydrates, the hepatic metabolism of fructose is very different to that of glucose in that it is insulin-independent, bypasses the process of glycolysis, and increases de novo lipogenesis to a greater extent; metabolically, fructose is similar to ethanol [120]. SSB consumption was recently reported to be associated with a risk of earlier menarche dose-dependently or -independently of childhood BMI [38,39].
Conclusions
In pediatrics, establishing risk factors is important in planning the prevention of disease in later life. In this review, we showed that early menarche increases the risk of a shorter final height and the development of obesity, IR, MetS, NAFLD, T2DM, breast cancer, and CVD in Korean women as with other ethnicities. Furthermore, a female adolescent with early menarche may suffer from various psychosocial problems and show delinquency and risky sexual behaviors. Although the above mentioned chronic cardiometabolic diseases might occur in adults and should be managed by all physicians, the pediatrician is an advance guard to recognize the problems correlated with early menarche and should take action to prevent its consequences.
We also reviewed the probable mechanism of early menarche: a high fructose intake, in the form of SSB and fruit juice (foods containing no fiber), is one of the major causes of early menarche, obesity, and IR.
A health education program that stresses a healthy diet composed mainly of unprocessed fruits (with much fiber like vegetables) and whole grains, should be implemented. Early menarche might be prevented by lifestyle changes, including regular exercise and a healthy diet (fructose-free and fiber-rich) during infancy and childhood. The secondary prevention of cardiometabolic disease in a female experiencing early menarche involves exercise and a healthy diet in line with obesity management.
We hope that this review will help pediatricians comprehensively understand the lifetime health problems associated with early menarche and to participate in preventing early menarche and its complications.





 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link PubMed
PubMed Download Citation
Download Citation


