Article Contents
| Clin Exp Pediatr > Volume 64(7); 2021 |
|
Abstract
Background
Purpose
Methods
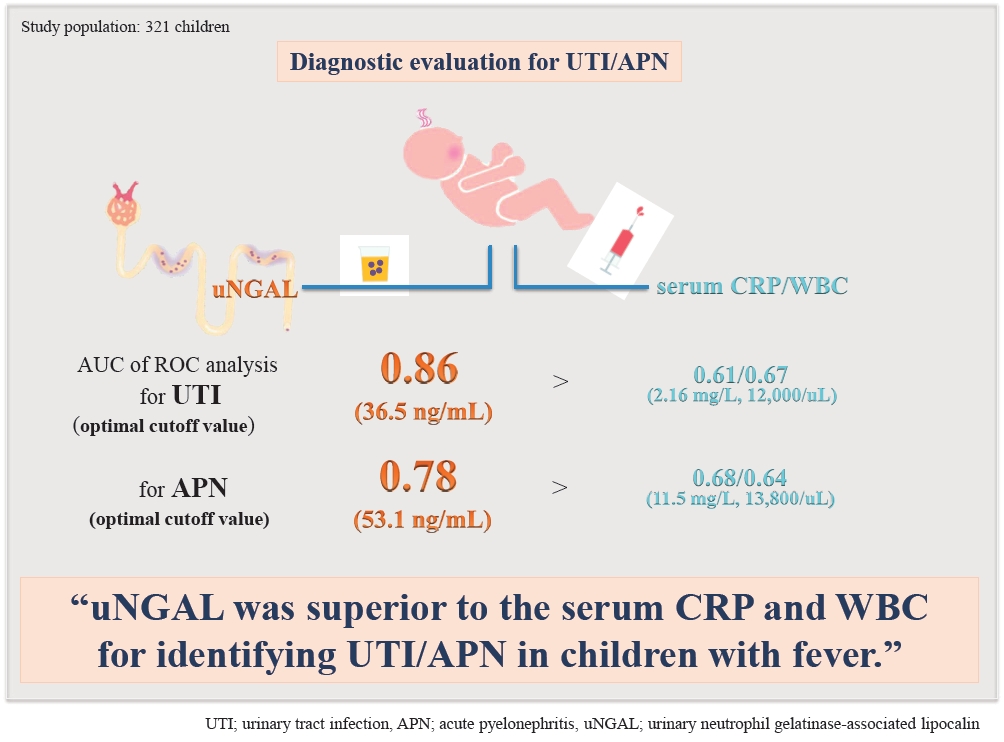
Results
Fig. 1.

Fig. 2.
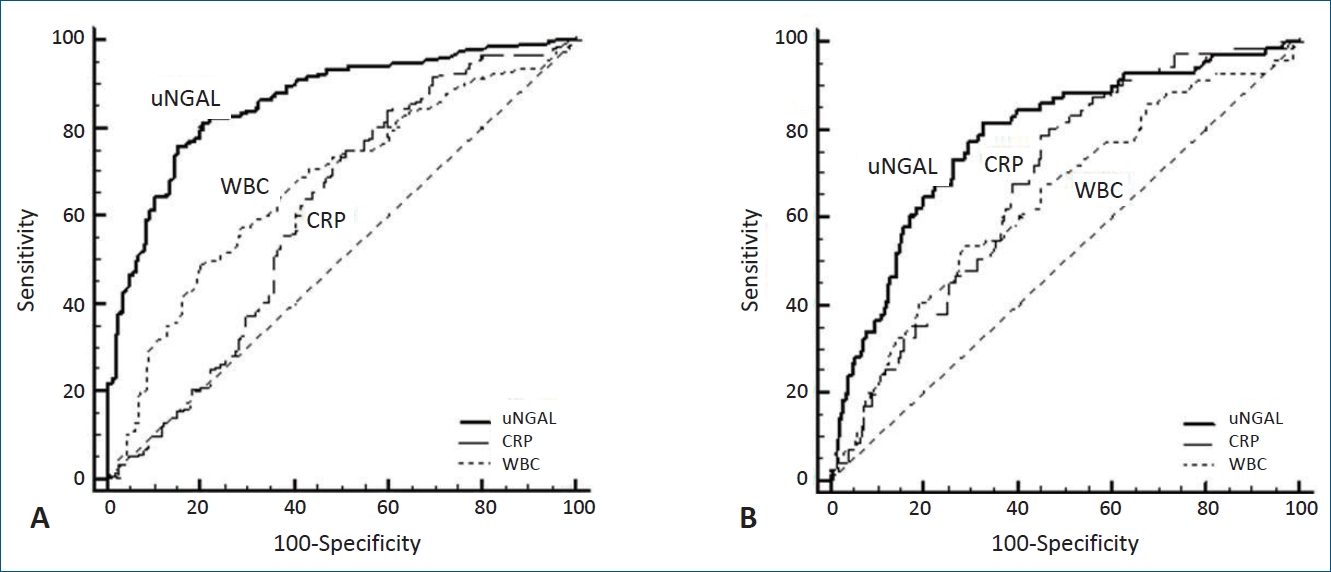
Table 1.
| Variable | Non-UTI (n=164) | UTI (n=157) | P value | Non-APN (n=87) | APN (n=70) | P value |
|---|---|---|---|---|---|---|
| Age (mo) | 60.3±62.3 | 16.8±33.7 | <0.001a) | 21.6±39.5 | 10.9±24.0 | 0.319a) |
| Female sex | 82/164 (50.0) | 57/157 (36.3) | 0.013a) | 31/87 (35.6) | 26/70 (37.1) | 0.845b) |
| Fever duration, >72 hr | 41/164 (25.0) | 18/157 (11.5) | 0.002b) | 9/87 (10.3) | 9/70 (12.8) | 0.624b) |
| WBC (μL) | 11,020±6,573 | 13,664±5,693 | <0.001a) | 13,088±5,335 | 14,381±6,071 | 0.213a) |
| Hb (g/dL) | 12.1±1.3 | 11.2±1.2 | <0.001c) | 11.4±1.3 | 11.1±1.2 | 0.172c) |
| CRP (mg/L) | 29.1±45.4 | 33.3±40.5 | 0.001a) | 23.7±32.6 | 45.3±46.0 | 0.001a) |
| Creatinine (mg/dL) | 0.4±0.2 | 0.2±0.1 | 0.001a) | 0.3±0.1 | 0.2±0.1 | 0.085a) |
| uNGAL (ng/mL) | 31.6±63.1 | 240.9±292.4 | <0.001a) | 200.1±270.7 | 291.6±311.8 | 0.014a) |
| Hydronephrosis | - | - | - | 60/87 (68.9) | 58/70 (82.8) | 0.076b) |
| VUR | - | - | - | 0/6 (0) | 17/69 (24.6) | 0.001b) |
Table 2.
| Variable |
Univariable |
Multivariable |
||
|---|---|---|---|---|
| Odds ratio (95% CI) | P value | Odds ratio (95% CI) | P value | |
| Age (mo) | 0.31 (0.21–0.46) | 0.001 | 0.49 (0.32–0.75) | 0.001 |
| Female sex | 0.57 (0.36–0.89) | 0.014 | 0.84 (0.47–1.48) | 0.534 |
| Fever duration, >72 hr | 0.39 (0.21–0.71) | 0.002 | 0.78 (0.54–1.18) | 0.364 |
| WBCa) | 1.11 (1.06–1.16) | 0.001 | 1.02 (0.97–1.08) | 0.395 |
| CRPa) | 1.02 (0.97–1.08) | 0.383 | - | - |
| uNGALa) | 1.16 (1.10–1.21) | 0.001 | 1.13 (1.08–1.18) | 0.001 |
| Hb | 0.59 (0.48–0.71) | 0.001 | 0.90 (0.69–1.18) | 0.453 |
UTI, urinary tract infection; CI, confidence interval; WBC, white blood cell; CRP, C-reactive protein; uNGAL, urinary neutrophil gelatinase-associated lipocalin; Hb, hemoglobin.
Table 3.
| Variable |
Univariable |
Multivariable |
||
|---|---|---|---|---|
| Odds ratio (95% CI) | P value | Odds ratio (95% CI) | P value | |
| Age (mo) | 0.96 (0.27–1.04) | 0.811 | - | - |
| Female sex | 0.79 (0.56–2.05) | 0.231 | - | - |
| Fever duration, >72 hr | 0.78 (0.48–3.42) | 0.150 | - | - |
| WBCa) | 1.04 (0.98–1.10) | 0.160 | - | - |
| CRPa) | 1.16 (1.06–1.28) | 0.002 | 1.01 (0.99–1.02) | 0.306 |
| uNGALa) | 1.12 (1.08–1.15) | 0.023 | 1.15 (1.04–1.27) | 0.038 |
| Hb | 0.83 (0.64–1.08) | 0.163 | - | - |
APN, acute pyelonephritis; CI, confidence interval; WBC, white blood cell; CRP, C-reactive protein; uNGAL, urinary neutrophil gelatinase-associated lipocalin; Hb, hemoglobin.
Table 4.
PPV, positive predictive value; NPV, negative predictive value; PLR, positive likelihood ratio; NLR, negative likelihood ratio; WBC, white blood cell; CRP, C-reactive protein; uNGAL, urinary neutrophil gelatinase-associated lipocalin; UTI, urinary tract infection; APN, acute pyelonephritis; AUC, areas under the curve; CI, confidence interval.
Boldface indicates a statistically significant difference with P<0.05.





 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link PubMed
PubMed Download Citation
Download Citation


