An otherwise healthy, 7-year-old female presented acutely with status epilepticus, aphasia, fever, encephalopathy, and prolonged Todd’s paralysis. The week prior to admission, the patient reported 2 self-limited generalized convulsions which did not require acute care. Labs on admission are displayed in
Table 1. The patient’s neuroimaging (
Fig. 1) revealed peri Rolandic and posterior parietal lobe restricted diffusion and cortical edema. Her electroencephalogram was abnormal as well, demonstrating cerebral slowing with left focal slowing. Of note, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) polymerase chain reaction was negative in the serum and cerebrospinal fluid although immunoglobulin G levels were not obtained. Following intervention with anticonvulsant therapy, she returned to her neurocognitive baseline and was discharged 2 days after admission.
The patient was readmitted the following week in with headaches, encephalopathy, abdominal pain, dysarthria/slurred speech, and altered mental status. Repeat magnetic resonance imaging brain showed previous diffusion restriction although cortical edema was less apparent. Labs are displayed in
Table 1 but on this admission, SARS-CoV-2 antibodies were checked, revealing a titer of 7.1. The patient was also found to be myelin oligodendrocyte glycoprotein (MOG) antibody positive with a titer of 1:100. For presumed postinfectious neurologic phenomenon, the patient was administered intravenous immunoglobulin 2 g/kg over 3 days. She showed improvement in her condition over 5 days and was discharged home with no further seizures. Upon follow-up, she had almost returned to her baseline with mild dysarthria.
Neurologic complications associated with SARS-CoV-2
Neurologic complications associated with SARS-CoV-2 have been reported with retrospective studies identifying between 14%–67% patients experience neurological disease during the acute infectious period [
1]. Reported neurologic phenomenon include anosmia, ageusia, seizures, meningoencephalitis, cerebrovascular disease, Guillain-Barré syndrome, postviral polyneuropathies, encephalitis, and acute disseminated encephalomyelitis (ADEM) particularly with hemorrhage (also termed acute necrotizing encephalitis) [
2]. Roughly 34% of children with SARS-CoV-2 related multisystem inflammatory syndrome in children have identified neurological manifestations, although these have generally been less severe phenotypes than observed in adults [
3]. Although encephalitis has been reported, this was in the setting of acute infection [
4], and thus far no postinfectious neurologic phenomenon have been reported.
Myelin oligodendrocyte glycoprotein (MOG) antibodies in children
In children, MOG antibodies are associated with a variety of postinfectious acquired demyelinating disorders, demonstrating a strong predilection for children. MOG antibodies have been identified over the last decade to be intimately linked with acquired demyelinating diseases with a strong predilection for striking in the pediatric age spectrum. Reported neurologic phenomenon associated with MOG antibodies include ADEM, optic neuritis, transverse myelitis, neuromyelitis optica, and autoimmune encephalitis. Although the exact etiology to MOG spectrum disorders is unknown, postinfectious molecular mimicry and epitope spreading are hypothesized. MOG antibody associated encephalomyelitis has been reported in 67% with infectious prodrome and have been related to postinfectious conditions including herpes simplex virus (HSV), Epstein-Barr virus (EBV), others [
5]. Armangue et al [
6]. have shown in their prospective study that HSV encephalitis can trigger autoimmune encephalitis in 27% of patients. SARS-CoV-2 being neurotropic might play similar role.
MOG and SARS-CoV-2
To date, there have been no associated cases of MOG spectrum disorders in children associated with either acute or postinfectious infection with SARS-CoV-2. Here we present a case of a 7-year-old female with a prior asymptomatic infection with SARS-CoV-2 who subsequently developed encephalopathy and status epilepticus in association with MOG antibody positivity. The presence of SARS-CoV-2 could be a coincidence, but a prior study has shown higher rates of nonencephalitic past HSV-1 infection significantly more in N-methyl-D-aspartate receptor encephalitis suggesting meaningful association between infection and the antibody mediated encephalitis [
7]. The phenotype in our case is characteristic of pediatric onset MOG spectrum disorders in that seizures, encephalopathy, and immunotherapy responsiveness are all reported. Although the patient’s radiographic findings are inconsistent with ADEM the clinical presentation of encephalopathy and seizure does match this disorder well, as does the presence of MOG antibodies. For this reason, the authors feel strongly that the patients presentation is associated with the presence of SARS-CoV-2 antibodies in spite of the patient having no occult respiratory illness reported by the family.
Mechanism of SARS-CoV-2 and autoimmune encephalitis
The exact mechanism is obscure whereas SARS-CoV-2 had been associated with autoimmune encephalitis in pediatric [
8], adults and the proposed mechanism of MOG antibodies presence is likely heterogeneous including molecular mimicry with identification of self antigens as foreign in the presence of similarity of epitopes, or initial infection leading to dispersion of central nervous system antigens to periphery with development of autoantibodies to myelin protein. Although restricted diffusion was present in our case, the radiographic findings are inconsistent with cerebrovascular disease or vasculopathy although the latter has been reported in persons with MOG antibodies and SARS-CoV-2 infection [
9].
Two important factors to neurologist
While singular cases are difficult to generalize to the greater population, there are 2 important factors of relevance to treating neurologists. Firstly, the potential link between prior SARS-CoV-2 infection and the subsequent development of neuroinflammatory disease is of importance given the high rates of infection around the globe. Although prevention of SARS-CoV-2 is of primary importance, understanding that MOG antibody spectrum disorders can occur in the postinfectious period is of importance. Further, amongst neurologists and pediatricians evaluating patients with new onset seizure activity or ADEM-like presentations, ascertainment of SARS-CoV-2 antibody status may be of importance from both a treatment and prognostication standpoint as postinfectious neuroinflammation tends to be monophasic [
10]. This is of greater importance in pediatric patients as there may be no occult respiratory phenomenon with initial infection.
Key message
Question: Although neurologic complications have been reported during the acute phase of infection in children, less is known about the postinfectious phenomenon associated with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus.
Finding: We present a case of a 7-year-old female with a prior asymptomatic infection with SARS-CoV-2 who subsequently developed encephalopathy and status epilepticus in association with myelin oligodendrocyte glycoprotein (MOG) antibody positivity.
Meaning: Here we present the first case of postinfectious of a MOG spectrum disorder associated with prior SARS-CoV-2 infection in a pediatric patient.
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Fig. 1.
(A) Axial T2 showing Left perirolandic cortex and posterior parietal lobe cerebral edema marked by the arrow. (B) Axial diffusion weighted image showing restricted diffusion marked by the arrow.
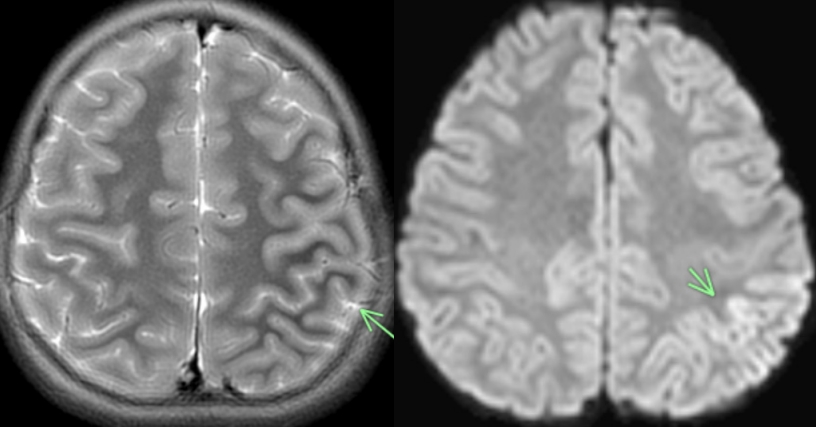
Table 1.
Pertinent lab findings from hospital admissions
|
Laboratory finding |
Hospital admission 1 |
Hospital admission 2 (+7 days) |
|
Serum |
|
|
|
MOG IgG |
1:40 |
1:100 |
|
SARS-CoV-2 PCR |
Negative |
Negative |
|
SARS-CoV-2 IgG |
Not obtained |
Positive (titer: 7.1) |
|
Aquaporin-4 antibody |
Negative |
Negative |
|
TNF-α |
Not obtained |
1.68 pg/mL (H) |
|
CSF |
|
|
|
White blood cell |
132 cell/mm3
|
116 cell/mm3
|
|
% lymphocytes |
64% |
90% |
|
Red blood cell |
1,000 cell/mm3
|
0 cell/mm3
|
|
Total protein |
51 mg/dL |
48 mg/dL |
|
Glucose |
73 mg/dL |
46 mg/dL |
|
Film arraya |
Negative |
Negative |
|
Mayo encephalitis panel |
Negative |
Negative |
|
Oligoclonal bands |
Not obtained |
Multiple OCB present in CSF/serum |
|
IgG index |
Not obtained |
0.55 |
References
6. Armangue T, Spatola M, Vlagea A, Mattozzi S, Cárceles-Cordon M, Martinez-Heras E, et al. Frequency, symptoms, risk factors, and outcomes of autoimmune encephalitis after herpes simplex encephalitis: a prospective observational study and retrospective analysis. Lancet Neurol 2018;17:760–72.


8. Burr T, Barton C, Doll E, Lakhotia A, Sweeney M. N-methyl-d-aspartate receptor encephalitis associated with COVID-19 infection in a toddler. Pediatr Neurol 2021;114:75–6.








 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link PubMed
PubMed Download Citation
Download Citation


