Article Contents
| Clin Exp Pediatr > Volume 64(11); 2021 |
|
Abstract
Background
Syncope is a common problem in children and adolescents. However, a large proportion of syncope cases have no underlying cause.
Purpose
This study aimed to identify the factors affecting the severity of syncope using tissue Doppler imaging (TDI).
Methods
This retrospective study included 61 children and adolescents with syncope who underwent echocardiography. The head-up-tilt test (HUT) was performed when there was a more severe syncopal event. We compared the echocardiographic findings between the execute HUT and nonexecute HUT, negative HUT result and positive HUT result, and normal electrocardiogram (ECG) and abnormal ECG groups. Data were analyzed using an unpaired t test post hoc analysis.
Results
In the execute and nonexecute HUT groups, the odds ratios were 0.55 for medial E/E′ (P=0.040) and 0.64 for lateral E/E′ (P=0.049). Comparison of the results of the decreased, normal, and increased groups for lateral E/E′ revealed a significant difference in the execution HUT and nonexecute HUT groups (overall, P=0.004; decreased vs. increased, P= 0.003; normal vs. increased, P=0.050).
Conclusion
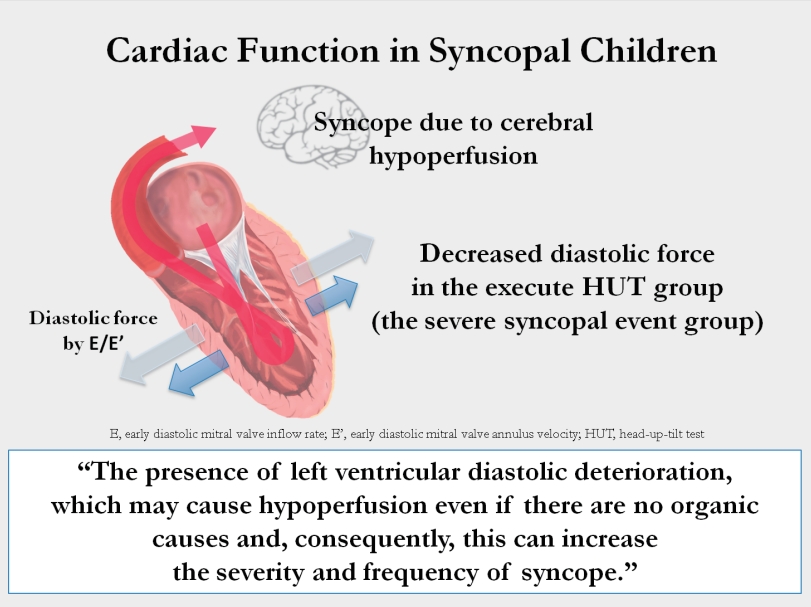
Medial E/E′ and lateral E/E′ were decreased in patients with severe syncopal events. These findings suggest that the presence of left ventricular diastolic deterioration may cause hypoperfusion even in the absence of organic causes and, consequently, increase syncope severity and frequency. The TDI measured by echocardiography can be used as an index to predict syncope recurrence and/or severity.
Graphical abstract.
The definition of syncope is characterized by cerebral hypoperfusion resulting in transient loss of consciousness and sudden, short, and spontaneous recovery [1]. Syncope is a common problem, and an estimated 15% of children experience one or more syncopal episodes until the end of adolescence [2]. The chief complaint of syncope accounts for 1% of all pediatric Emergency Department visits [3].
Syncope can be classified as reflex syncope, orthostatic hypotension, and cardiac syncope. The major cause of syncope is reflex syncope, which is caused by an inappropriate reflex [1]. Reflex syncope includes vasovagal, situational, carotid sinus syndrome, and nonclassic forms, and it is a common clinical condition occurring even in otherwise healthy people without an underlying cardiovascular disease [4]. Regardless of the underlying etiology, multiple studies confirmed that syncope decreases one’s quality of life to a degree similar to other chronic illnesses [3]. Symptoms are blacking out, dizziness, falling down, fainting, visual disturbance, and so on. Thus, even clinically benign vasovagal episodes may end in a fatal event for the individual or for third parties when occurring in hazardous conditions [5]. Furthermore, syncopal symptoms in children and adolescents can inhibit not only their school attendance but also their ability to participate in extracurricular activities. Syncopal events that cannot be predicted escalate anxiety for the affected individual, resulting in further isolation [2].
A syncopal episode diagnosed as reflex syncope without a specific underlying cause would negatively affect children and adolescents’ lives as aforementioned. Therefore, we aimed to identify the factors affecting the incidence and severity of syncope through echocardiography.
In this retrospective study, 113 children and adolescents aged 10 to 17 years who visited Gangnam Severance Hospital for a syncopal event from January 2015 to May 2018 were screened. Among these patients, 67 patients visited the pediatric cardiology outpatient clinic, those with an underlying disease, with no echocardiographic evaluation, or with echocardiographic abnormalities were excluded; thus, 61 patients were enrolled (Fig. 1).
Instead of using conventional Doppler echocardiography for estimating conventional left ventricular (LV) inflow, we used tissue Doppler imaging (TDI) to quantitatively evaluate LV systolic and diastolic myocardial movements. Even if the LV ejection fraction (LVEF) is normal, if partial myocardial function decreases, it can be detected sensitively through TDI (Fig. 2) [6].
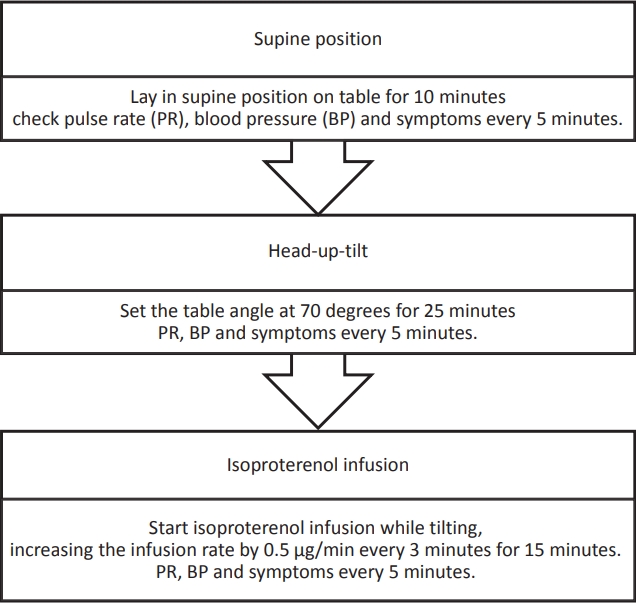
The head-up-tilt test (HUT) was executed according to the following criteria: (1) Presence of a recurrent syncopal episode, (2-1) high risk of physical damage (head trauma), or (2-2) hazardous event (ex. athletes and cases in hazardous places such as swimming pools), even though it was a single event, and (3) Difficult to distinguish between reflex syncope and orthostatic hypotension. The division of risk groups was based on the 2018 European Society of Cardiology guideline [1].
HUT was executed in 3 stages (supine, head-up-tilting, and isoproterenol infusion) according to the severance hospital protocol (Fig. 3). The results of the HUT were classified as positive or negative. The cardioinhibitory type, vasodepressor type, and mixed type, which belong to reflex syncope, were classified as positive [7]. We diagnosed vasodepressor type when blood pressure (BP) decreased by more than 30% from initial BP, cardioinhibitory type when HR decreased by more than 20%, and mixed type when both BP down and HR down were included.
The electrocardiogram (ECG) rhythm was divided into normal or abnormal. Abnormal ECGs included a borderline prolonged QT, first-degree atrioventricular (AV) block, premature atrial contraction (PAC), and infrequent premature ventricular contraction. The QTc of the patients with prolonged QT interval was all less than 480 msec, so it was considered as borderline QT prolong. In addition, the patients had no past and family history other than syncope, so we did not classify it separately from other abnormal ECG group.
All the measured echocardiographic parameters were compared between the execute and nonexecute HUT groups, positive and negative HUT groups, and normal and abnormal ECG groups. Mean values of age and echocardiographic parameters were compared between each group, and odds ratios (ORs) were used to verify the correlation. We used multivariate logistic regression analysis and included age to determine the OR and adjust for the effect of age on TDI [8,9].
The cutoff value was applied to the significantly identified parameters to verifying the difference between the groups. Cutoff values for medial E/E’ were decreased medial E/E’ (<5.3), normal medial E/E’ (5.3–8.5), and increased medial E/E’ (>8.5). Cutoff values for lateral E/E’ were decreased lateral E/E’ (<3.8), normal lateral E/E’ (3.8–6.4), and increased lateral E/E’ (>6.4). The cutoff value of E/E' was not applied differently according to age and sex [10].
In addition, some patients underwent electroencephalogram (EEG), but executing criteria for EEG was not specific and none of the patients showed abnormal findings, so EEG result was not included in the analysis.
Statistical analyses were performed using IBM SPSS Statistics ver. 23.0 (IBM Co., Armonk, NY, USA). Data were analyzed using the unpaired t test to obtain mean and variance, and the OR with 95% confidence interval was calculated in a multivariate logistic regression model. The mean difference between the 3 groups was analyzed by post hoc analysis using analysis of variance. In all analyses, a P value less than 0.05, was considered to be statistically significantly different.
The total number of patients was 61, and the mean age was 14.1 years. The proportions of boys and girls were 37.7% and 62.3%, respectively. Of the 32 patients underwent HUT, 20 were confirmed as negative, and of the 12 patients who were positive (62.5%), 11 patients had the vasodepressor type (34.4%), 1 patient was mixed type (3.1%), and 0 patients were of cardioinhibitory type. Most patients had a normal ECG (54, 88.6%), and in the abnormal ECG group, borderline prolonged QT (3, 4.9%), PAC (2, 3.3%), right bundle branch block (1, 1.6%), and first-degree AV block (1, 1.6%) were confirmed. No patient had LVEF less than 53% (Table 1).
We divided all the echocardiographic parameters into 2 groups and compared the mean of each group. Age (13.50±1.83 years vs. 14.61±1.87 years, P=0.017), LV end-systolic diameter (29.00±3.01 mm vs. 30.91±4.08 mm, P=0.041), medial E/E' (7.41±1.68 vs. 6.70±0.96, P=0.044), and lateral E/E' (5.55±1.25 vs. 4.90±0.91, P=0.029) of were significantly different between the nonexecute HUT and execute HUT groups. In addition, age (13.95±2.04 years vs. 15.62±0.96 years, P=0.017) was significantly different between the negative HUT and positive HUT groups. Lateral A’ (6.68±1.73 cm/sec vs. 5.36±1.29 cm/sec, P=0.021) and lateral S’ (7.38±2.29 cm/sec vs. 5.82±0.87 cm/sec, P=0.031) of were significantly different between the normal ECG and abnormal ECG groups (Table 2).
For each variable, the OR was calculated by multivariate logistic regression analysis that included age to control for agedependent variables. As a result, the ORs of medial E/E 'and lateral E/E' were 0.55 and 0.64 in the comparison between the execute HUT and nonexecute HUT groups (Table 3). Further, we divided medial E/E' and lateral E/E', which were significant values in multivariate logistic regression, into 3 groups, normal, decreased, and increased. Cutoff values of medial E/E’ are decreased (<5.3), normal (5.3–8.5), increased (>8.5), and that of lateral E/E’ are decreased (<3.8), normal (3.8–6.4), increased (>6.4). Post hoc analysis of age, HUT, HUT result, and ECG result was performed to determine if there was a difference in average values between each group [10]. There was no significant difference in medial E/E’ between each group. However, in the case of lateral E/E', we identified that there is a correlation between this indicator and whether HUT executed or not (overall P=0.004). In each comparison, there was a significant difference between increased and decreased groups (P=0.003), and between normal and increased groups (P=0.05).
HUT was performed when the syncope occurred repeatedly or when there was a high-risk event, and the medial E/E' and especially the lateral E/E' were decreased in children who underwent HUT. No variables were associated with HUT results, which suggest that the type of syncope is not related to cardiac function. According to Shen et al. [11], when isoproterenol is used, the sensitivity of the HUT increases, so it is useful in combination with the tilting test. The positive result was confirmed at 11.7±3.6 minutes after isoproterenol injection. In our study, the time taken to confirm positive was 7.75±5.03 minutes.
E/E' is one of the earliest indicators of LV diastolic dysfunction. According to Yu et al. [6], the early diastolic velocity of the mitral annulus (E') reflects myocardial relaxation. E' increases when the transmitral gradient increases as the exercise or preload increases. However, if there is a problem with myocardial relaxation, E' decreases. The decrease in E' is one of the earliest markers reflecting diastolic dysfunction. E velocity represents transmitral velocity. As the LV filling pressure (LVFP) increases due to diastolic dysfunction, E velocity increases and E' velocity decreases. E/E' presents LVFP or pulmonary capillary wedge pressure (PCWP).
According to Kadappu and Thomas [12], septal E/E' >15 or lateral E/E' >12 is as marker of elevated LVFP. Especially, E/E' >15 is an independent predictive marker of cardiac mortality and heart failure. However, it is debatable whether mitral E/E' is the reliable indicator of LV diastolic dysfunction. In a study of healthy subjects by Previtali et al. [13], PCWP was well associated with mitral E velocity but not with septal and lateral E/E' ratios. The lateral E/E' ratio correlates well with LVEDP only when LV function is depressed. Additionally, the reference range of E/E' for children and adolescents is not precisely defined. The reference value of TDI is affected by race, age, and sex, and the E/E' value decreases in adolescence as one’s age increases [8,10].
Symptoms of syncope are induced by cerebral hypoperfusion, although there are differences in the mechanism depending on the type of syncope. It may occur when a decrease in cardiac output occurs, such as vasodilatation, bradycardia, hypotension, and LV diastolic dysfunction [14]. Studies about the relationship between LV diastolic dysfunction and syncope have been reported. Samdani et al. [15] reported that patients with syncope had LV diastolic dysfunction compared to those without syncope by using TDI. Yamaguchi et al. [16] suggested that LV dysfunction has a worse prognosis in patients with vasovagal syncope.
In this study, we found that in the patients with no underlying disease with normal cardiac function, subtle differences in cardiac function that were confirmed by measuring TDI could affect the severity of syncope. However, this difference did not reflect E/E' values, which were significantly increased to 15 or more as revealed in previous studies, because patients were limited to those with normal cardiac function. In patients with syncope, a sensitive index of TDI, even within the normal range of echocardiographic findings, revealed a slight difference in LV diastolic function in patients with severe syncope. In addition to the limitations of evaluating the severity of syncope through TDI in this study, there are also limitations of TDI itself. In the case of TDI, since the information on the wall motion is provided only in 1 dimension, the information on the wall motion in 3 dimension is insufficient, and can be affected by changes in heart rate and loading conditions [17]. These limitations can cause TDI to vary in results depending on the timing of the test and the examiner. Despite these limitations, TDI is a very important tool for evaluating cardiac systolic and diastolic function [6].
The limitations of this study are that it was performed in a single institution, the number of patients was small, not all patients who visited an Emergency Department with syncope came to the Pediatric Cardiac Outpatient Clinic, and we could not perform echocardiography in all outpatients so the selected patient group could be biased. We retrospectively analyzed patients who had experienced syncope and could not compare the echo parameters with normal patients who had no syncope events. Therefore, prospective, big data studies will be needed to compare the results of echocardiography with those patients who have or have not experienced syncope. In addition, in measuring the echocardiographic parameter TDI, which may show a variation depending on the situation, it is necessary to repeatedly perform echocardiography for more accurate results. The normal range of E/E' in adolescents has not yet reached a consensus, so it is necessary to establish the normal E/E' value. Further, since the E/E' value changes with age, it is necessary to conduct a more detailed investigation of this age group of patients in a large-scale study.
In conclusion, medial E/E' and lateral E/E', which represent LV diastolic function, were different between patients with severe or frequent syncopal events and those without. These findings suggest that patients with no abnormal cardiac function and no organic causes experienced recurrent and/or severe syncopal events when there were subtle changes in LV diastolic function compared with their counterparts. We thought that these differences can affect the frequency and severity of syncope by making subtle deterioration in cardiac function, which should be confirmed by prospective big data studies. Through further research, the TDI measured by echocardiography may be used as an index to predict recurrence and/or severity of syncope.
Fig. 1.
Flow diagram of the syncope patient evaluation. ED, Emergency Department; OPD, out patient department.
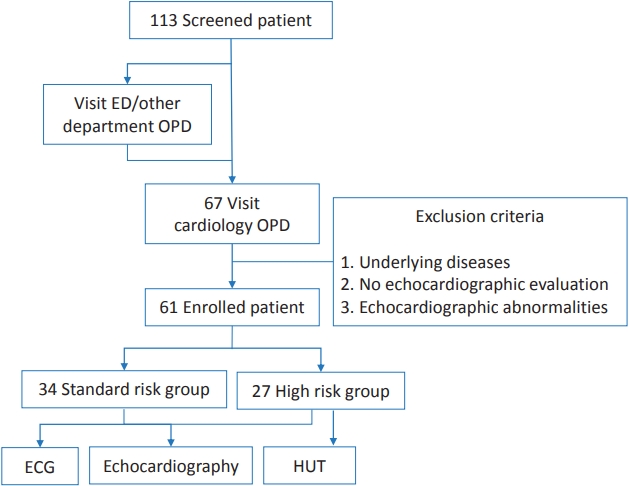
Fig. 2.
Conventional and tissue Doppler echocardiographic measurements. RV, right ventricular; LV, left ventricular; RA, right atrium; LA, left atrium.
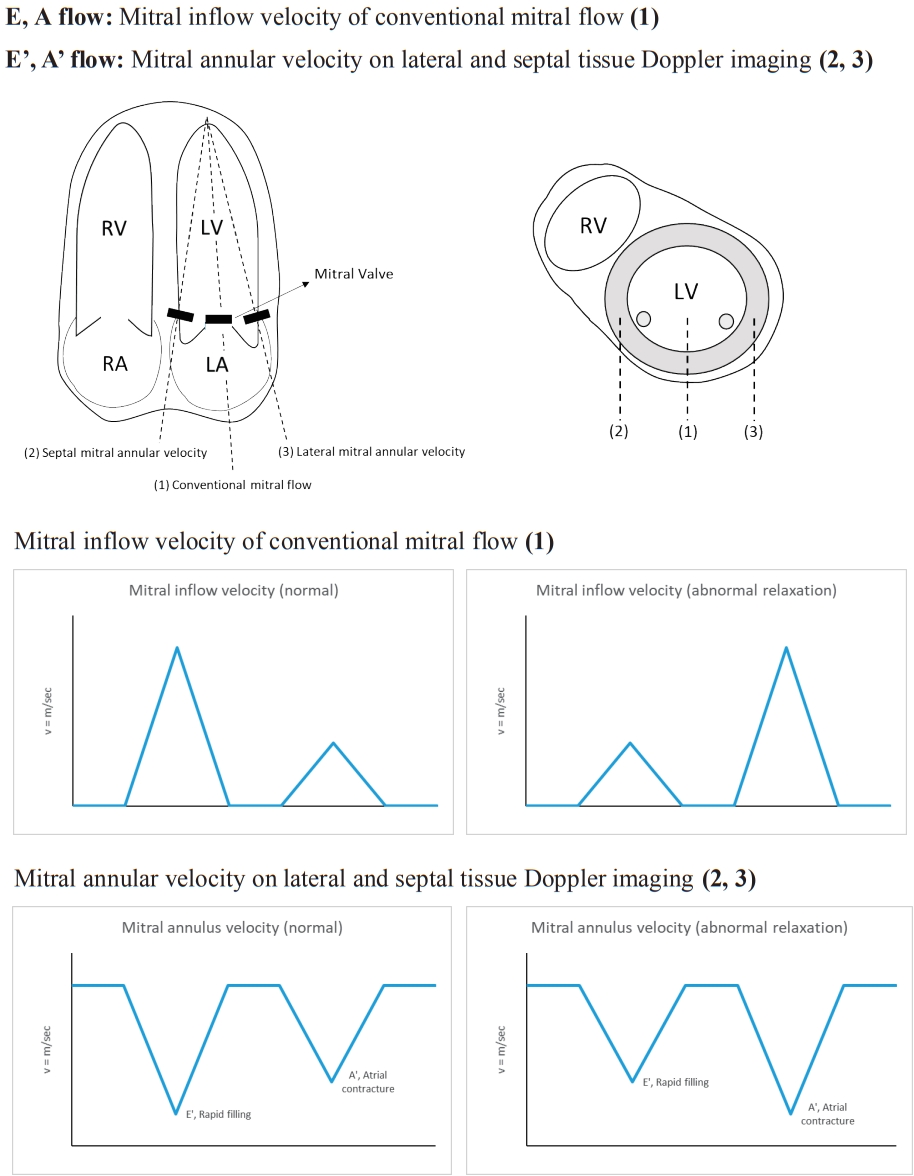
Table 1.
Patient characteristics (n=61)
Table 2.
Left ventricular parameters of the execute and nonexecute HUT, negative and positive HUT, and normal and abnormal ECG groups
Values are presented as mean±standard deviation. The data were analyzed using t-tests.
HUT, head-up-tilt test; ECG, electrocardiogram; LVEF, left ventricular ejection fraction; LVFS, left ventricular fractional shortening; LVEDD, left ventricular enddiastolic diameter; LVESD, left ventricular end-systolic diameter; DT, deceleration time; E, early diastolic velocity of conventional Mitral inflow Doppler velocity; A, late diastolic velocity of conventional Mitral inflow Doppler velocity; E', early diastolic tissue Doppler Mitral annular velocity; A', late diastolic tissue Doppler Mitral annular velocity; S', systolic tissue Doppler Mitral annular velocity.
Boldface indicates a statistically significant difference with P<0.05.
References
1. Brignole M, Moya A, de Lange FJ, Deharo JC, Elliott PM, Fanciulli A, et al. 2018 ESC Guidelines for the diagnosis and management of syncope. Eur Heart J 2018;39:1883–948.


2. Friedman KG, Alexander ME. Chest pain and syncope in children: a practical approach to the diagnosis of cardiac disease. J Pediatr 2013;163:896–901.



3. Anderson JB, Willis M, Lancaster H, Leonard K, Thomas C. The Evaluation and Management of Pediatric Syncope. Pediatr Neurol 2016;55:6–13.


4. Fu Q, Levine BD. Pathophysiology of neurally mediated syncope: role of cardiac output and total peripheral resistance. Auton Neurosci 2014;184:24–6.



5. Barbic F, Dipaola F, Casazza G, Borella M, Minonzio M, Solbiati M, et al. Syncope in a working-age population: recurrence risk and related risk factors. J Clin Med 2019;8:150.



6. Yu CM, Sanderson JE, Marwick TH, Oh JK. Tissue Doppler imaging a new prognosticator for cardiovascular diseases. J Am Coll Cardiol 2007;49:1903–14.

7. Raddino R, Zanini G, Robba D, Bonadei I, Chieppa F, Pedrinazzi C, et al. Diagnostic value of the head-up tilt test and the R-test in patients with syncope. Heart Int 2006;2:171.



8. Choi SH, Eun LY, Kim NK, Jung JW, Choi JY. Myocardial tissue Doppler velocity in child growth. J Cardiovasc Ultrasound 2016;24:40–7.



9. Dallaire F, Slorach C, Hui W, Sarkola T, Friedberg MK, Bradley TJ, et al. Reference values for pulse wave Doppler and tissue Doppler imaging in pediatric echocardiography. Circ Cardiovasc Imaging 2015;8:e002167.


10. Caballero L, Kou S, Dulgheru R, Gonjilashvili N, Athanassopoulos GD, Barone D, et al. Echocardiographic reference ranges for normal cardiac Doppler data: results from the NORRE Study. Eur Heart J Cardiovasc Imaging 2015;16:1031–41.


11. Shen WK, Jahangir A, Beinborn D, Lohse CM, Hodge DO, Rea RF, et al. Utility of a single-stage isoproterenol tilt table test in adults: a randomized comparison with passive head-up tilt. J Am Coll Cardiol 1999;33:985–90.

12. Kadappu KK, Thomas L. Tissue Doppler imaging in echocardiography: value and limitations. Heart Lung Circ 2015;24:224–33.


13. Previtali M, Chieffo E, Ferrario M, Klersy C. Is mitral E/E' ratio a reliable predictor of left ventricular diastolic pressures in patients without heart failure? Eur Heart J Cardiovasc Imaging 2012;13:588–95.


14. Wieling W, Thijs RD, van Dijk N, Wilde AA, Benditt DG, van Dijk JG. Symptoms and signs of syncope: a review of the link between physiology and clinical clues. Brain 2009;132(Pt 10):2630–42.

15. Samdani AJ, Samdani H, Mohammed M, Kattel S, Meesala M, Saito Y. Left ventricular diastolic dysfunction is more common in patients with syncope. J Am Coll Cardiol 2015;65(10_Supplement): A434.






 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link PubMed
PubMed Download Citation
Download Citation


