Article Contents
| Clin Exp Pediatr > Volume 65(9); 2022 |
|
Abstract
Background
In 2013, the Thai Pediatric Oncology Group (ThaiPOG) introduced a national protocol in which high-dose chemotherapy plus stem cell rescue is performed without immunotherapy.
Methods
This study aimed to elucidate the outcomes of high-risk neuroblastoma (HR-NB) patients treated with the ThaiPOG protocol. This retrospective cohort review included 48 patients (30 males, 18 females) with a median age of 3 years (range, 8 months to 18 years) who were treated at 5 ThaiPOG treatment centers in Thailand in 2000–2018.
Results
Eight of the 48 patients showed MYCN amplification. Twenty-three patients (48%) received 131I-meta-iodobenzylguanidine prior to high-dose chemotherapy and stem cell rescue. The majority of patients achieved a complete or very good response prior to consolidation treatment. The 5-year overall survival (OS) and event-free survival (EFS) rates were 45.1% and 40.4%, respectively. Patients aged >2 years had a nonsignificantly higher mortality risk (hazard ratio [HR], 2.66; 95% confidence interval [CI], 0.92–7.68; P=0.07). The MYCN amplification group had lower OS and EFS rates than the MYCN nonamplification group, but the difference was not statistically significant (45% OS and 37.5% EFS vs. 33.3% OS and 16.6% EFS; P=0.67 and P=0.67, respectively). Cis-retinoic acid treatment for 12 months was a strong prognostic factor that could reduce mortality rates among HR-NB patients (HR, 0.27; 95% CI, 0.09–0.785; P=0.01).
Graphical abstract
High-risk neuroblastoma (HR-NB) is the most common extracranial solid tumor in children. The standard treatment in developed Western countries consists of induction chemotherapy, surgery, consolidation with high-dose chemotherapy, stem cell rescue, radiotherapy, immunotherapy with anti-GD2 antibody, and differentiation therapies with isotretinoin [1]. Most patients with HR-NB in countries with limited resources have poor access to immunotherapy [2]. While the survival rate of patients in high-income countries is 50%–80%, the 4-year survival rate of patients with HR-NB treated without autologous stem cell transplant (ASCT) or immunotherapy has been reported to range from 20% to 30% [2,3].
In 1998, Thai Pediatric Oncology (ThaiPOG) established a national treatment protocol for childhood cancers, including HR-NB. The first HR-NB national protocol consisted of 3 treatment phases, namely, induction, consolidation, and maintenance therapy with multiagent chemotherapy combined with surgery and irradiation. A single intuitional study reported 1-, 2-, and 3-year overall survival (OS) rates of 33%, 18.5%, and 14.8%, respectively, in 27 patients with HR-NB [4]. Survival results are markedly inferior compared with the treament outcome reports during the same period [5,6].
Thailand’s current national protocol for HR-NB consists of topotecan-based induction chemotherapy, which has been reported to lead to excellent tumor responses with acceptable toxicity [7]. Consolidation therapy may be performed in 2 ways: (1) chemotherapy with ifosfamide, carboplatin, and etoposide for 4 cycles and (2) high-dose chemotherapy plus stem cell rescue. Introduction of high-dose chemotherapy plus stem cell rescue to the treatment protocol has significantly improved the 5-year event-free survival (EFS) of patients with HR-NB [8-11]. A study in India on ASCT using melphalan and/or busulfan as a conditioning regimen demonstrated a 3-year EFS of 39% [12].
Treatment selection is often based on family socioeconomics because high-dose chemotherapy plus stem cell rescue is not covered by the universal healthcare system in Thailand. Thus, high-dose MIBG treatment, i.e., 12–18 mCi per kg, is incorporated during the conditioning regimen prior to high-dose chemotherapy plus stem cell rescue only if the family could afford the treatment [13].
The objective of our study is to elucidate the outcome of HR-NB treated according to ThaiPOG’s national protocol with high-dose chemotherapy plus stem cell rescue without immunotherapy.
A retrospective review of patients with HR-NB treated with high-dose chemotherapy plus stem cell rescue at 5 ThaiPOG treatment centers from 2000 to 2018 was performed in this work. The study was approved by the Institutional Review Board (IRB 668/63, Faculty of Medicine, Chulalongkorn University, Bangkok, Thialand) and performed according to the ethical principles outlined in the Declaration of Helsinki (1975) and its revision. ThaiPOG defines HR-NB as (1) The International Neuroblastoma Staging System (INSS) stage 4 in children older than 18 months; (2) stage 4 disease, age younger than 18 months with MYCN amplification, or (3) any age or stage with MYCN amplification.
Patients who were diagnosed prior to 2013 were treated with 4 cycles of induction chemotherapy, as provided in the ThaiPOG98 regimen. Each cycle consists of 60 mg/m2 cisplatin given for 1 day, 100 mg/m2 etoposide given for 2 days, 30 mg/m2 doxorubicin given as a single dose, and 900 mg/m2 cyclophosphamide given daily for 2 days. Patients who were diagnosed after 2013 were given topotecan-based treatment consisting of intravenous 1.2 mg/m2 topotecan combined with 400 mg/m2 cyclophosphamide daily for 5 days in the first 2 induction cycles. The third and fifth induction cycles comprised 50 mg/m2 intravenous cisplatin given daily for 4 days combined with 200 mg/m2 etoposide given daily for 3 days. The fourth and sixth induction cycles included 2,100 mg/m2 intravenous cyclophosphamide given daily for 2 days combined with 25 mg/m2 doxorubicin given daily for 3 days and 0.67 mg/m2 vincristine given daily for 3 days. Each cycle was administered at 28-day intervals, regardless of count recovery. Granulocyte-colony stimulating factor (G-CSF) was prophylactically administered after every cycle.
Responses to induction therapy were categorized according to the international neuroblastoma response criteria as complete (CR), very good partial response (VGPR), partial response, mixed response, no response, or progressive disease at primary and metastatic sites.
Peripheral blood stem cell harvest for ASCT (frozen) is recommended after completing the second or third course of induction chemotherapy in cooperation with G-CSF priming. The surgical intervention for total primary removal was recommended after the fourth and fifth cycles of induction chemotherapy. Recommended 131I-meta-iodobenzylguanidine (MIBG) treatment dose is 18 mCi/kg in a single visit at 4-6 weeks before ASCT.
Recommended high-dose chemotherapy regimen comprises busulfan 37.5 mg/m2 every 6 hours for 4 days, followed by melphalan 70 mg/m2 given daily for 2 days. Stem cell infusion with the recommended dose of CD34+ cell 1-5×105 cell/kg will be infused after high-dose chemotherapy completion. G-CSF was routinely administered after stem cell infusion until white blood cells engraftment. Maintenance therapy with isotretinoin is given after ASCT once the patient is discharged and hematological recovery with the dose of 80 mg/m2 twice daily (160 mg/m2/day) for 14 consecutive days every 4 weeks. The version of the national protocol decides the duration of isotretinoin therapy for 6–12 months.
Demographic data and complications after ASCT were analyzed using frequency and percentage for categorical data and mean and standard deviation for continuous data. The survival rates were described by using the Kaplan–Meier method and log-rank test. The definition of survival time is from the date of diagnosis. Cox regression analysis was applied and hazard ratios (HRs) with 95% confidence intervals (CIs) were analyzed to describe the effect of several variables, including age at diagnosis, sex, MYCN status, induction regimen, residual disease, MIBG treatment, conditioning regimen and also duration of cis-retinoic acid treatment, on survival rates. All analyses were performed using Stata 10.1 (Stata Corp., College Station, TX, USA). All test statistics were 2-sided, and a P value of less than 0.05 was considered statistically significant.
A total of 48 (30 males, 18 females) patients with HR-NB who underwent high-dose chemotherapy plus stem cell rescue or ASCT as consolidation therapy from 5 tertiary centers from 2000 to 2018, were enrolled in our study (Table 1). The median age at diagnosis was 3 years (range, 8 months to 18 years). An 18-year-old female patient was diagnosed with stage IV neuroblastoma without MYCN amplification. All patients were determined to be INSS stage IV. The MYCN status of 14 patients was available, and 8 of 14 patients (57%) demonstrated MYCN amplification. The majority of the patients (60.4%) received topotecan and cyclophosphamide, and the remaining patients received the ThaiPOG98 regimen as an induction chemotherapy protocol. Twenty-three patients (48%) received MIBG treatment prior to high-dose chemotherapy plus stem cell rescue. Thirty patients (62.5%) achieved CR/VGPR prior to consolidation treatment. According to the induction regimen, there was no difference in the response rate between ThaiPOG98 and Topo/cyclo regimen (P=0.106).
Nearly all patients (46 of 48, 95.8%) received single ASCT. Peripheral blood was the major source of stem cells in our cohort. Approximately 80% of the patients received a busulfan–melphalan regimen for conditioning. The median time of neutrophil engraftment was 11 days (range, 6–52 days), and the median time of platelet engraftment was 24 days (range, 8–156 days). No difference in neutrophil and platelet engraftment was found between the busulfan and melphalan (BuMel) and carboplatin etoposide and melphalan (CEM) groups. One patient who received BuMel with peripheral blood stem cell rescue did not achieve platelet engraftment and relapsed within 5 months after transplantation.
Nearly all patients (43 of 48) received oral cis-retinoic acid as a postconsolidation therapy. Cis-retinoic (80 mg/m2) was administered for 14 consecutive days for each 28-day cycle. A total of 16 patients received cis-retinoic acid treatment for 6 months, while 27 patients received the same treatment for 12 months. The treatment duration was decided by the patients’ care provider. No difference in age, MYCN status, or induction response was noted between the 6- and 12-month cis-retinoic treatment groups. Additional treatment modalities, including surgery, chemotherapy, radiotherapy and MIBG treatment, were applied for adjunctive therapy. Two patients received a second ASCT, but this procedure did not appear to provide survival benefits to those with relapsed disease (Table 2).
After ASCT, 33 of the 48 patients (68.7%) relapsed, with a median follow-up time of 46 months (range, 9–164 months). Moreover, 32 of the 48 patients (66.7%) died from the disease; 1 patient died of unknown causes. The median OS and EFS time were 4.8 and 3.2 years, respectively. The 5-year OS and EFS were 45.1% and 40.4%, respectively (Fig. 1).
The version national protocol decided the duration of isotretinoin therapy. The first version was recommended for 6 months based on published data. Among this group of 6 months isotretinoin, only 3 patients relapsed during cis-retinoic acid, all 3 patients were alive. They continued cis-retinoic until the completion of 6 months of therapy, and 13 patients relapsed after stopping cis-retinoic acid, with a median time of 3.5 months after containing cis-retinoic acid (range, 0–33 months). The following version of the national protocol recommends at least 12 months of isotretinoin therapy from this observation.
Prognostic factors that may affect outcomes, including age at diagnosis, sex, MYCN oncogene, induction regimen, MIBG treatment before ASCT, residual disease, conditioning regimen, and cis-retinoic acid treatment, were evaluated, as shown in Table 3. We observed that patients aged over 2 years had a higher mortality risk when compared with younger patients; however, the difference between these patients was not statistically significant (HR, 2.66; 95% CI, 0.92–7.68; P=0.07). Cis-retinoic acid treatment for 12 months was a strong prognostic factor that could reduce mortality among patients with HR-NB (HR, 0.27; 95% CI, 0.09–0.785; P=0.01).
OS and EFS according to different prognostic factors are shown in Fig. 2. Patients aged less than 2 years at diagnosis had better 5-year OS and EFS (63.6% and 60.6%, respectively) when compared with patients aged over 2 years (38.5% and 35.1%, respectively). MYCN status was available for only 14 patients. Poorer OS and EFS were found in the MYCN amplification group when compared with the nonamplification groups (45% [OS], 37.5% [EFS] vs. 33.3% [OS], 16.6% [EFS], respectively). The BuMEL group had better OS and EFS than the CEM group (52.8% [OS], 44.4% [EFS] vs. 0% [OS], 20% [EFS], respectively). In Thailand, consolidated therapy is limited to cisretinoic acid. Groups who received cis-retinoic acid treatment for 12 months showed significantly better outcomes than those who received treatment for only 6 months (60.4% [OS], 51.8% [EFS] vs. 20.0% [OS], 25.0% [EFS], respectively; P=0.00).
The majority of the ASCT complications observed was febrile neutropenia. Only 10% of all patients had documented infection, and one-third of all patients developed severe mucositis. Severe mucositis and veno-occlusive disease, particularly in the BuMel group, were also noted. Liver injury and kidney complications were found only in the CEM group. No patient died from ASCT complications (Supplementary Table 1). Thus, cis-retinoic acid is generally well tolerated, and good adherence with no serious adverse effect was reported.
This study is the first to report the outcomes of patients with HR-NB following consolidation therapy with high-dose chemotherapy plus stem cell rescue in Thailand. Because this treatment modality is not covered by the universal healthcare system of Thailand, only 48 patients received high-dose chemotherapy plus stem cell rescue as a part of their treatment.
Compared with previous reports, our study demonstrated much higher OS and EFS rates. The 5-year OS and EFS in our study were 43.1% and 34.8%, respectively, and the median OS and EFS times were 4.8 and 3.2 years, respectively. A previous report on the outcomes of 27 patients with HR-NB revealed a 3-year OS of 14.8% and median survival time of 9 months [4]. Besides intensive consolidation therapy, the superior outcomes of our cohort may be attributed to the use of advanced multimodality treatment, including MIBG treatment, cis-retinoic acid, irradiation, surgical technique, and general supportive care.
The patients’ age at diagnosis affected treatment outcomes. Better outcomes were observed among patients aged less than 2 years compared with older patients even though there was no statistically significant in our study due to the limited number of patients. We believe that this finding may be attributed to older patients commonly having molecular markers of poor prognosis, such as deletion of chromosome 19p [14]. Younger neuroblastoma patients may be good candidates for ASCT in countries with limited resources.
Our results are comparable with the treatment outcomes in settings with limited resources but far inferior compared with those of the Western world [12,15]. This difference may be attributed to the use of postconsolidation maintenance therapy, which is immunotherapy plus cis-retinoic acid, is the current standard of care. Our study demonstrated that cis-retinoic acid monotherapy during the maintenance phase significantly affects EFS and OS rates. Maintenance therapy for 12 months demonstrated superior OS and EFS compared with maintenance therapy for 6 months. Given these results, 12 months of cisretinoic acid therapy was incorporated into our current national protocol.
Disease relapse in patient without gross residual disease occurred after discontinuation of cis-retinoic acid. Our next study will assess whether cis-retinoic acid treatment exceeding 1-year results in longer disease- and event-free periods, especially in settings where immunotherapy is inaccessible.
No treatment-related mortality from high-dose chemotherapy plus stem cell rescue was noted, and treatment-related complications were found in only a small number of patients. Cis-retinoic acid is generally well tolerated, and all of the patients received treatment according to their recommended treatment plans.
In conclusion, high-dose chemotherapy plus stem cell rescue follow by cis-retinoic acid for 12 months is well tolerated and could improve survival in patients with HR-NB.
This report presents a number of limitations that may affect the interpretation of the results. First, we enrolled patients whose MYCN status could not be evaluated; thus, the data may not be sufficiently strong to elucidate the maximum effect of MYCN amplification. Second, functional imaging, such as MIBG scan or positron emission tomography-computed tomography scan, was not available. Thus, assessing the full effects of the disease on survival is challenging. Further prospective neuroblastoma studies including more prognostic factors and complete functional imaging should be performed to improve treatment outcomes.
Supplementary materials
Supplementary Table 1 can be found via https://doi.org/10.3345/cep.2022.00437.
Supplementary Table 1
Adverse events that occurred during autologous stem cell transplantation (N = 48)
Acknowledgments
The authors gratefully acknowledge the English Editing Service, Research Affairs, Faculty of Medicine, Chulalongkorn University for the English language editing assistance.
Fig. 1.
Five-year overall survival (OS; A) and event-free survival (EFS; B) rates among neuroblastoma patients.
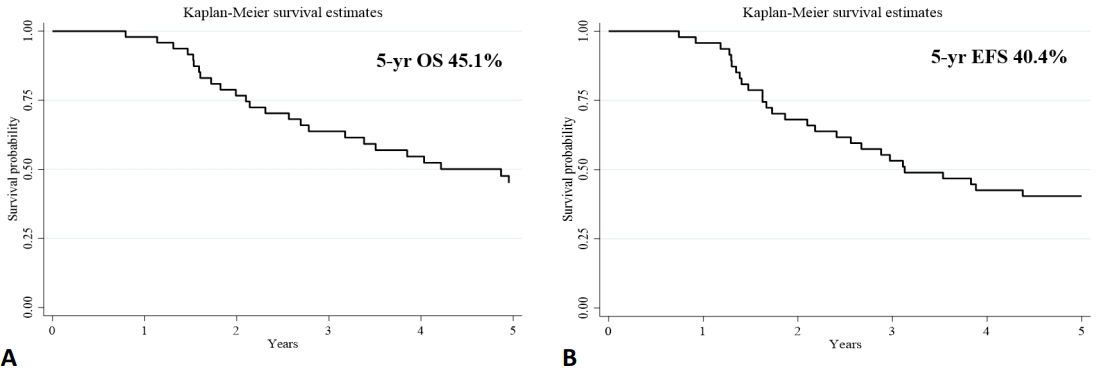
Fig. 2.
Overall survival (OS; A) and event-free survival (EFS; B) rates after maintenance of cis-retinoic acid for 6 versus 12 months.

Table 1.
Characteristics of patients with high-risk neuroblastoma (N = 48)
Values are presented as median (range) or number (%).
INSS, International Neuroblastoma Staging System; MIBG, 131I-metaiodobenzylguanidine; ASCT, autologous stem cell transplantation; CR, complete response; VGPR, very good partial response; PR, partial response; BuMel, busulfan and melphalan; CEM, carboplatin etoposide and melphalan; TNC, total nucleated cell.
Table 2.
Treatment modalities after autologous stem cell transplantation (N=48) modified to cis/non-cis with adjunctive treatment
Table 3.
Prognostic factors and overall outcomes (univariate analysis)
| Factor | Crude HR (95% CI)a) | P valuea) |
|---|---|---|
| Age (yr) | 0.07 | |
| ≤2 | 1 | |
| >2 | 2.66 (0.92–7.68) | |
| Sex | 0.35 | |
| Male | 1 | |
| Female | 1.4 (0.68–2.87) | |
| MYCN oncogene | 0.34 | |
| No amplification | 1 | |
| Amplification | 1.26 (0.33–4.74) | |
| Induction regimen | 0.32 | |
| ThaiPOG98 | 1 | |
| Topo/Cyclo | 1.44 (0.69–3.03) | |
| MIBG treatment before ASCT | 0.47 | |
| Not done | 1 | |
| Done | 1.29 (0.64–2.60) | |
| Disease before ASCT | 0.77 | |
| Response (CR, VGPR, PR) | 1 | |
| Nonresponse | 0.85 (0.29–2.49) | |
| Conditioning regimen | 0.17 | |
| BuMel | 1 | |
| CEM | 2.13 (0.71–6.36) | |
| Cis-retinoic acid | 0.01 | |
| No | 1 | |
| 6 Months | 0.76 (0.26–2.17) | |
| 12 Months | 0.27 (0.09–0.78) |
HR, hazard ratio; CI, confidence interval; Topo/Cyclo, topotecan plus cyclophosphamide; MIBG, 131I-meta-iodobenzylguanidine; ASCT, autologous stem cell transplantation; CR, complete response; VGPR, very good partial response; PR, partial response; BuMel, busulfan and melphalan; CEM, carboplatin etoposide and melphalan.
References
1. Yu AL, Gilman AL, Ozkaynak MF, London WB, Kreissman SG, Chen HX, et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. New Engl J Med 2010;363:1324-34.



2. Jain R, Trehan A, Menon P, Kapoor R, Kakkar N, Radhika S, et al. Survival in patients with high-risk neuroblastoma treated without autologous stem cell transplant or dinutuximab beta. Ped Hematol Oncol 2021;38:291-304.

3. Haghiri S, Fayech C, Mansouri I, Dufour C, Pasqualini C, Bolle S, et al. Long-term follow-up of high-risk neuroblastoma survivors treated with high-dose chemotherapy and stem cell transplantation rescue. Bone Marrow Transplant 2021;56:1984-997.



4. Wiangnon S, Jetsrisuparb A, Komvilaisakm P, Kamsaaad S. Survival rate of children with neuroblastoma in Srinagarind hospital. Srinagarind Med J 2003;18:154-9.
5. Lau L. Neuroblastoma: a single institution’s experience with 128 children and an evaluation of clinical and biological prognostic factors. Ped Hematol Oncol 2002;19:79-89.

6. Pannelli F, Mosciatti P, Felici L, Magnani C, Pascucci C, Pastore G. Survival trends of childhood cancer during the period 1978-1994 in Italy: a first report from the Italian cancer registries. Epidemiol Prevenz 2001;25:354-75.
7. Rujkijyanont P, Photia A, Traivaree C, Monsereenusorn C, Anurathapan U, Seksarn P, et al. Clinical outcomes and prognostic factors to predict treatment response in high risk neuroblastoma patients receiving topotecan and cyclophosphamide containing induction regimen: a prospective multicenter study. BMC Cancer 2019;19:961.




8. Matthay KK, Villablanca JG, Seeger RC, Stram DO, Harris RE, Ramsay NK, et al. Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid. Children’s Cancer Group. New Engl J Med 1999;341:1165-73.


9. Matthay KK, Reynolds CP, Seeger RC, Shimada H, Adkins ES, Haas-Kogan D, et al. Long-term results for children with high-risk neuroblastoma treated on a randomized trial of myeloablative therapy followed by 13-cis-retinoic acid: a Children’s Oncology Group study. J Clin Oncol 2009;27:1007-13.



10. Berthold F, Boos J, Burdach S, Erttmann R, Henze G, Hermann J, et al. Myeloablative megatherapy with autologous stem-cell rescue versus oral maintenance chemotherapy as consolidation treatment in patients with high-risk neuroblastoma: a randomised controlled trial. Lancet Oncol 2005;6:649-58.


11. Pritchard J, Cotterill SJ, Germond SM, Imeson J, de Kraker J, Jones DR. High dose melphalan in the treatment of advanced neuroblastoma: results of a randomised trial (ENSG-1) by the European Neuroblastoma Study Group. Ped Blood Cancer 2005;44:348-57.

12. Jain R, Hans R, Totadri S, Trehan A, Sharma RR, Menon P, et al. Autologous stem cell transplant for high-risk neuroblastoma: achieving cure with low-cost adaptations. Ped Blood Cancer 2020;67:e28273.


13. Group TTPO. National protocol for the treatment of childhood Cancer 2018. In: Wiangnon S, editor. National protocol for the treatment of childhood cancer 2018. Bangkok (Thailand): Sahamitr Printing and Publishing, 2018:237-47.





 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link PubMed
PubMed Download Citation
Download Citation


