Article Contents
| Clin Exp Pediatr > Volume 66(9); 2023 |
|
Abstract
Hearing in neonates and infants is crucial for their development of language and communication skills. Unless hearing loss is appropriately managed early, it can cause a significant socioeconomic burden considering its detrimental impact on the child's development and its common nature. It is also the most common congenital sensory deficit, with an approximate incidence of 1.5 per 1,000 newborns. Its etiologies are heterogeneous: genetic causes are reportedly involved in up to 80% of cases, while congenital cytomegalovirus infection is the leading environmental factor contributing to congenital hearing loss. The introduction of newborn hearing screening using automated auditory brainstem response and/or automated otoacoustic emission in many developed countries has helped detect and manage hearing loss early. Current auditory rehabilitation options such as cochlear implantation implementing cutting-edge technologies can treat almost all degrees of hearing loss, emphasizing the importance of early hearing detection and intervention. Rapidly developing genetic diagnostic technologies and future cutting-edge treatment options, including gene therapy, will shed light on the future management of hearing loss in neonates and infants.
Graphical abstract. NICU, neonatal intensive care unit.
Hearing loss in infancy and childhood can cause delayed speech and language skills, thus creating a substantial socioeconomic cost [1,2]. The linguistic and communicative abilities of children with hearing loss can be maximized by early detection and rehabilitation to ensure their successful integration into a hearing society [3,4]. As social awareness of hearing loss grows among the general public, medical system and related medical innovations to support hearing loss are being developed. The adoption of a screening method for congenital hearing loss has created a paradigm shift in the diagnosis and treatment of hearing loss in neonates and infants [5,6]. Here we review the prevalence, etiologies, and screening protocols for hearing loss in neonates and infants.
The incidence of congenital hearing loss in the literature is approximately 1.5 per 1,000 newborns. In detail, those figures were 1.33 and 1.86 per 1,000 newborns with various etiologies in the United Kingdom and United States (Fig. 1) [7]. In Korea, the prevalence of hearing loss was 1.2 per 1,000 newborns according to a paper published after the government-supported nationwide newborn hearing screening (NHS) pilot project [8]. NHS became available as a health insurance benefit in October 2018 in Korea, which considerably increased the percentage of newborns who underwent NHS. Only 55.4% (1,741,556 of 3,141,384) of newborns underwent NHS in 2010–2016 [9] versus 91.7% (280,868 of 306,273) in 2019 and 92.1% (254,199 of 276,105) in 2020 [10].
Hearing loss is notably more common in premature infants or those admitted to the neonatal intensive care unit (NICU). According to previous reports, hearing loss was diagnosed in 1.2%–11% of preterm neonates depending on gestational age [11-13]. Newborns admitted to the NICU are at a 1.6%–13.7% increased risk of developing hearing loss [14-17]. A recent study in Korea reported that, whereas 4.6 of every 1,000 healthy infants were estimated to have hearing loss, the number jumped to 28.8 of every 1,000 newborns hospitalized for more than 5 days in the NICU [18].
The national incidence of congenital hearing loss varies among ethnicities and diagnostic processes, and accurate statistics are difficult to obtain. The implementation of a nationwide newborn hearing loss screening program permitted the early detection of congenital hearing loss and helped determine its incidence in many developed countries [7,19].
The prevalence of hearing loss increases gradually through infancy and adolescence, affecting an estimated 1.07–2.7 per 1,000 children aged 3–4 years versus 2.05–3.5 per 1,000 adolescents [7,20]. According to a study from Korea, infants with suspected acquired or delayed hearing loss, after passing the NHS, underwent initial audiology exams followed by cochlear implantation, which was performed for auditory rehabilitation on patients with severe to profound hearing loss, significantly later than those in the “refer” group [9]. The early and accurate detection of hearing loss in neonates and children is essential because permanent hearing loss at this age, which increases over that period, imposes considerable language development delays, communication issues, and social costs. It was recently suggested that pure-tone audiometry be performed on a regular basis around the age of 5 years or during adolescence to avoid a delayed diagnosis of hearing loss, especially the late-onset or progressive type [21].
Although many factors contribute to hearing loss in neonates and children (Fig. 1), there are 2 major players in the etiology of congenital hearing loss: genetic and environmental. Congenital hearing loss was traditionally believed to have a hereditary component in approximately 50% of cases, while another 25% were considered idiopathic and the rest remained unknown. According to reports based on ongoing genetic research, up to 80% of congenital hearing loss cases in developed nations are caused by genetic factors, with the remaining 20% caused by environmental or acquired factors (Fig. 2) [22,23]. Environmental factors include prenatal TORCH (toxoplasmosis, syphilis, rubella, cytomegalovirus, and herpes) infection or postnatal bacterial meningitis [24]. Congenital cytomegalovirus infection is gradually becoming recognized as the predominant environmental cause of congenital hearing loss, particularly in developed countries including the Republic of Korea [7,22,25].
Genetic causes include syndromic and nonsyndromic features as well as autosomal dominant/recessive/X-linked or mitochondrial inheritance patterns. Among them, nonsyndromic and autosomal recessive patterns outnumber syndromic and autosomal dominant patterns. This indicates that, in many cases, congenital hearing loss occurs with no family history or other clinical features, which could be an unexpected issue for family members. Recently, several studies investigated the adjunctive genetic screening of universal newborn hearing screening (UNHS); however, a mean 1.4% of children passed the UNHS but had a positive genetic screening test result [26,27].
Table 1 shows the most frequent causes of syndromic and nonsyndromic hearing loss [28,29]. The most prevalent type of congenital deafness in developed nations is DFNB1 (the first mapped locus for nonsyndromic deafness with autosomal recessive inheritance), which is caused by mutations in the GJB2 gene (gap junction protein beta 2) [30]. It encodes connexin 26 (also known as gap junction beta 2). In the cochlea, connexin 26 is expressed between the supporting cells and stria vascularis, spiral ligament, and spiral limbus. It appears to be involved in the potassium recycling process that hair cells utilize to produce an action potential in response to sound waves [31]. Therefore, it is widely accepted that GJB2 gene mutations cause sensorineural hearing loss. Pendred syndrome, the most prevalent syndromic hearing loss, is caused by mutations in the SLC26A4 (solute carrier family 26 member 4) gene on chromosome 7, which produces the pendrin protein [32]. A child must inherit 2 mutated SLC26A4 genes from each parent to have Pendred syndrome because of its recessive nature. Hearing loss in Pendred syndrome is frequently, although not always, present from birth, generally worsens over time, and is associated with minor head trauma [33]. Enlarged vestibular aqueducts, which make the inner ear sensitive to trauma, are characteristic of this syndrome. Vertigo can also occur. Goiter is a defining characteristic of this syndrome, occurring in 75% of cases.
The concept of UNHS for newborns was first suggested by Larry Fisch in 1957 [34]. However, it was not until the end of the 20th century that it became a standard trend in almost every developed country. In Korea, NHS were introduced in obstetrics and gynecology departments in the early 2000s; in 2007–2008, an NHS project supported by the government started in some regions, and it has gradually expanded. It eventually became covered by the national health insurance in 2018.
NHS were initially performed only in high-risk groups with risk factors for hearing loss. However, only approximately half of all infants diagnosed with hearing loss had a risk factor for hearing loss, with the remaining half of cases occurring in infants lacking risk factors [35]. Most developed countries currently conduct hearing screenings for all newborns. The UNHS administers a combination of 2 screening tests—automated auditory brainstem response (AABR) and automated otoacoustic emission (AOAE)—to newborns within 28 days of life. UNHS for premature infants is performed according to corrected age calculated based on the expected date of birth. The sensitivity and specificity of AOAE are reportedly 50%–100% and 13%– 91%, respectively, while those of AABR are 96% and 98%, respectively [36]. The NHS protocol is applied separately in Korea for “well babies,” those who were born through an uncomplicated delivery and stayed less than 4 days in the NICU, and “NICU babies,” who stayed for more than 5 days in the NICU and required treatment [37]. All neonates who stayed in the NICU for more than 5 days were considered at high risk for hearing loss. Additionally, the outpatient protocol should be used for newborns who have not undergone NHS or have meningitis or other diseases that increase their risk of hearing loss despite passing the screening test. The most recent update of the NHS protocol in Korea is shown in Figs 3–5. The high-risk factors for hearing loss suggested by the 2019 guidelines of the American Joint Committee on Infant Hearing are presented in Table 2 [4]. Regardless of the NHS results, newborns with these risk factors should be referred to the otolaryngology department for comprehensive hearing evaluation and regular checkups.
Once an infant passes the NHS, the hearing status is repeatedly checked using questionnaires implemented for infant medical checkups through 71 months of age since audiometric tests are not routinely performed. Some etiologies of hearing loss can cause late-onset or progressive hearing loss that initially presents as mild hearing loss and may not be detected on the NHS. Thus, hearing tests using pure-tone audiometry around 5 years of age are currently performed in many developed countries [38] and the introduction of childhood hearing screenings is under active discussion in Korea.
For example, auditory neuropathy spectrum disorder (characterized by signal processing dysfunction along the auditory nerve or compromised signal transmission to the auditory nerve by presynaptic inner hair cells with fully functional outer hair cells) can be easily missed when only AOAE is performed; thus, it is necessary to consider that a missed diagnosis is possible even after UNHS.
Although the UNHS has been implemented quite efficiently in developed nations since its inception, inequalities persist globally. While some countries, such as Ireland and the Netherlands, boast a screening rate of over 99%, many countries have not yet implemented it [39]. An analysis of review articles of the coverage of newborn and infant hearing screening programs is shown in Fig. 6 [39,40]. Approximately one-third of countries (38% of the world’s population) offered little or minimal screening, while another third (33% of the world’s population) offered UNHS programs that were nearly or fully implemented. Compared to countries with less than 10% screening coverage, the average living standard in almost fully or fully screened countries is 10 times higher [39].
In addition, with the rapid development of sequencing technologies, molecular genetic testing may compensate for the pitfalls of UNHS. However, this is not currently feasible because of the many uncertainties that would arise in the interpretation of the test results.
In Korea, the predicted referral rate for neonates undergoing NHS in 2020 is 0.99%–1.43% depending on the calculation method, while the proportion of infants finally diagnosed with congenital hearing loss has not yet been identified. However, the frequency of diagnostic hearing tests required for reference results in the NHS remains low. More than 97% of newborns born in 2020 received their first NHS within 7 days of birth, but only 4.3% underwent their first diagnostic auditory brainstem response by 3 months of age as recommended, while 82.3% underwent the test after 6 months.
With the advent of hearing screening protocols and the development of various auditory rehabilitation options, hearing loss is no longer an incurable disease, and many etiologies have been elucidated with the development of genetic diagnostic techniques. If the diagnosis of hearing loss is made before 3 months of age and auditory rehabilitation is provided at the appropriate time, an infant’s hearing ability and language development could be comparable to those of normal hearing children [41]. Based on these findings, the importance of the 1-3-6 rule could not be emphasized further: a hearing screening no later than 1 month of age, a diagnosis of hearing loss no later than 3 months of age, and entry into early intervention services by no later than 6 months of age. Transient auditory deprivation during critical periods is detrimental to the development of central auditory function [42], while the implementation of an Early Hearing Detection and Intervention program is necessary.
Sensorineural hearing loss is the most common sensory deficit globally, and its various etiologies include genetic and environmental causes. The introduction of NHS enabled the early detection of hearing loss, in turn facilitating early intervention. Many treatment options, including hearing aids and cochlear implants, based on the etiologic diagnosis, can rehabilitate mild to profound hearing loss. With recently developed genetic diagnostic techniques and the possibility of gene therapy, expectations for the early diagnosis and treatment of hearing loss are higher than ever before.
Fig. 1.
Estimates of causes of deafness at birth and 4 years of age in the United States. The incidence of deafness at birth in the United States and its prevalence at 4 years of age were obtained by adjusting estimates from the United Kingdom to include unilateral hearing loss. EVA, enlarged vestibular aqueduct; CMV, cytomegalovirus. Reprinted from Morton and Nance. N Engl J Med 2006;354:2151-64, with permission of Massachusetts Medical Society [7].
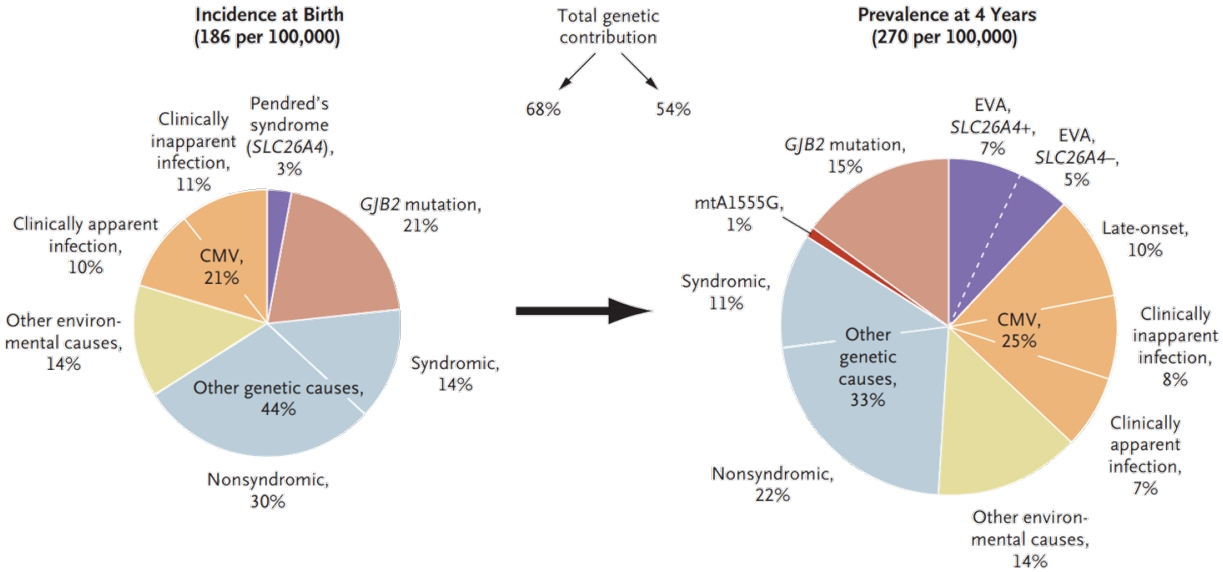
Fig. 2.
Approximate causes of congenital hearing loss. Recent genetic studies suggest that up to 80% of congenital hearing loss cases in wealthy nations are genetically based. This figure was traditionally assumed to be around 50%. Environmental factors usually involve prenatal TORCH (toxoplasmosis, syphilis, rubella, cytomegalovirus, and herpes) infection or postnatal bacterial meningitis.
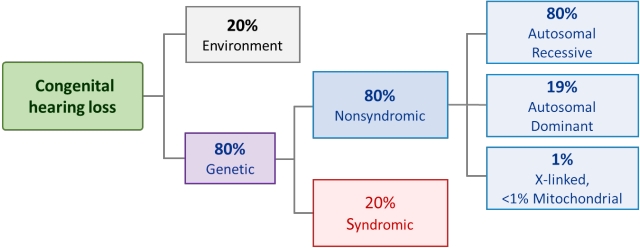
Fig. 3.
Well-baby newborn hearing screening protocol of the newborn nursery unit. This protocol applies to neonates born via an uncomplicated delivery who stayed less than 4 days in the neonatal intensive care unit. *Risk factors are described in Table 2. ABR, auditory brainstem response; NHS, newborn hearing screening. Adapted from Newborn hearing screening guideline update in Korea, 2018 by The Korean Audiological Society [37].
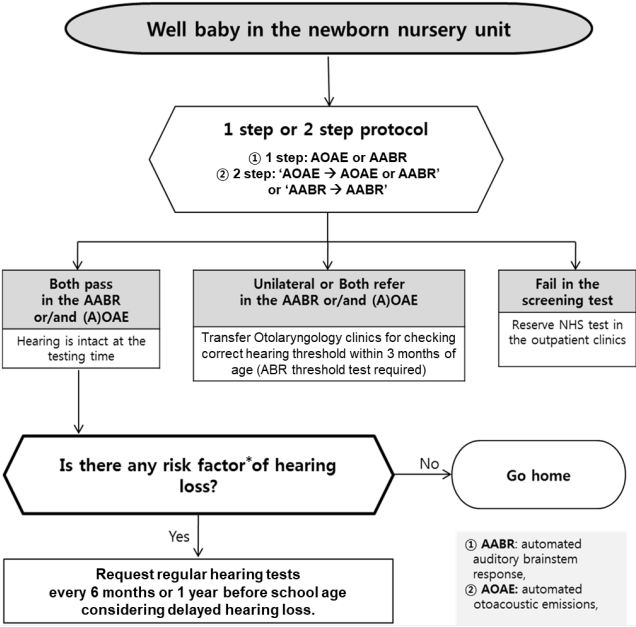
Fig. 4.
Neonatal intensive care unit (NICU) newborn hearing screening protocol. The corrected age is always used. Because a NICU admission longer than 5 days is already a high-risk factor for hearing loss, regular hearing tests are necessary for NICU babies. *Risk factors are described in Table 2. NICU, neonatal intensive care unit; AABR, automated auditory brainstem response; ABR, auditory brainstem response; AOAE, automated otoacoustic emission; NHS, newborn hearing screening. Adapted from Newborn hearing screening guideline update in Korea, 2018 by The Korean Audiological Society [37].
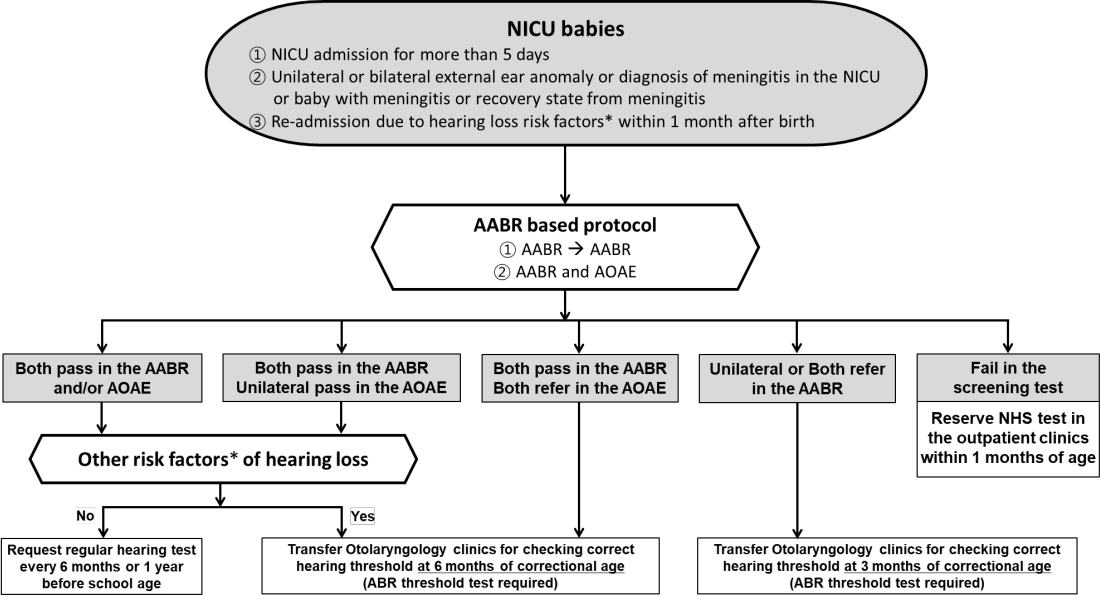
Fig. 5.
Newborn hearing screening protocol used in outpatient clinics. *Risk factors are described in Table 2. NICU, neonatal intensive care unit. Adapted from Newborn hearing screening guideline update in Korea, 2018 by The Korean Audiological Society [37].
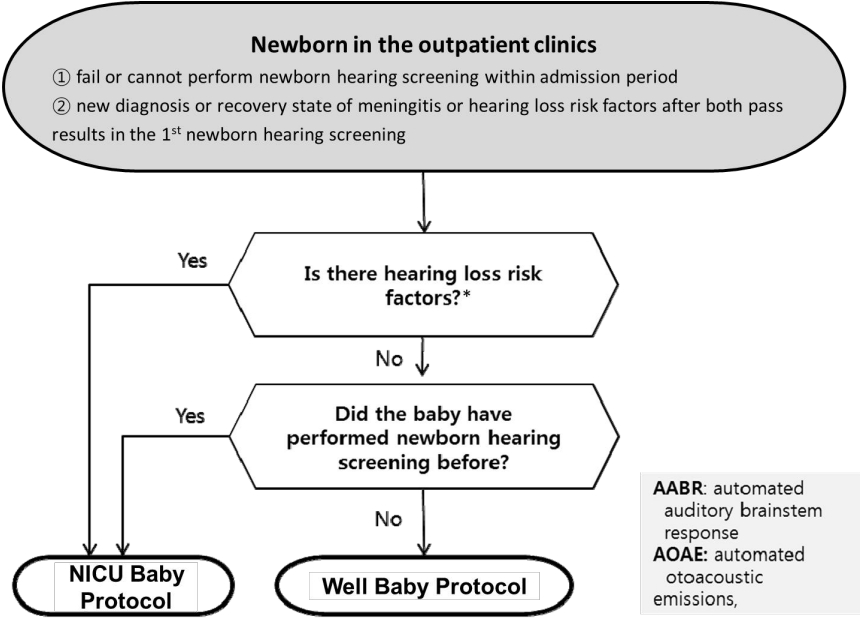
Fig. 6.
Country-specific coverage of newborn and infant hearing screening programs. Adapted from Neumann et al. J Clin Med 2022;11:271 [40].
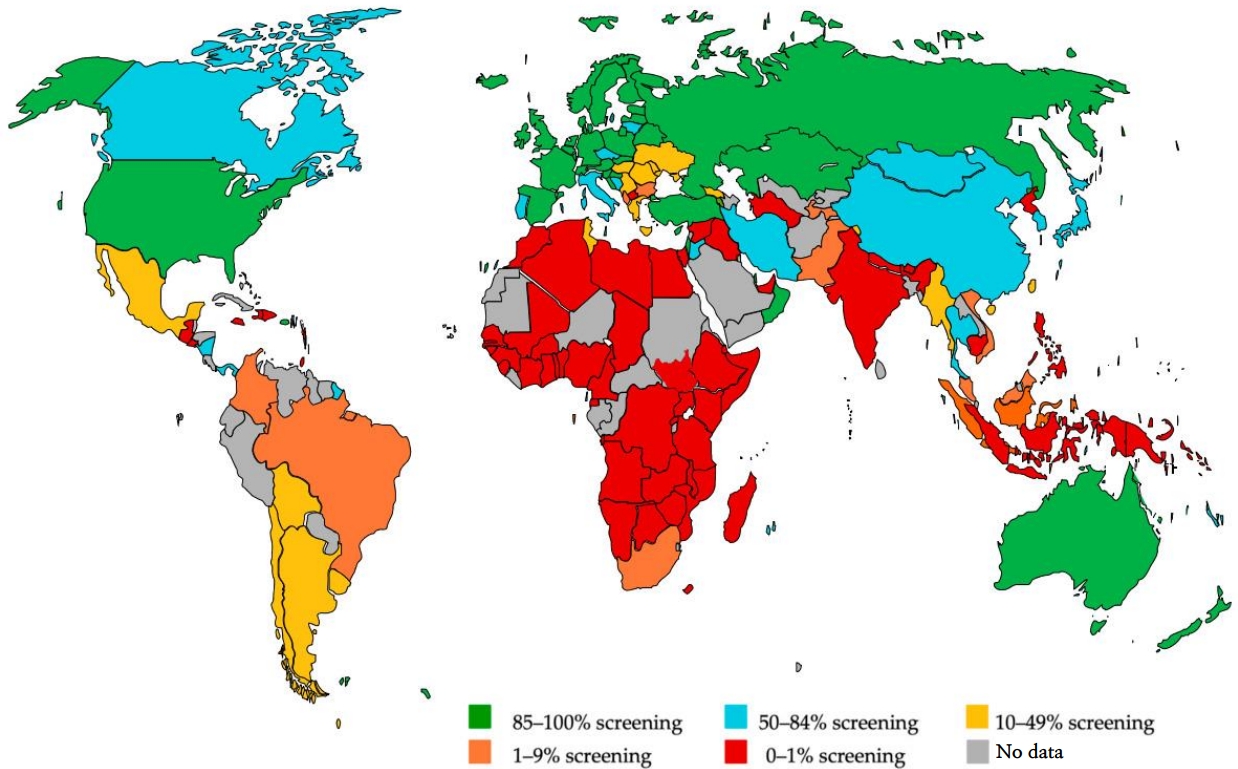
Table 1.
Frequent etiologies of syndromic and nonsyndromic hearing loss
CDH23, cadherin-related 23; COCH, cochlin; GJB2, gap junction protein beta 2; KNCQ4, potassium voltage-gated channel subfamily Q member 4; MELAS, mitochondrial encephalomyopathy, lactic acidosis, and strokelike episodes; MERRF, myoclonic epilepsy with ragged red fibers; MYO15A, myosin XVA; OTOF, otoferlin; SLC26A4, solute carrier family 26 member 4; TECTA, tectorin alpha; TMC1, transmembrane channel like-1; WFS1, wolframin ER transmembrane glycoprotein.
Table 2.
Risk factors associated with permanent hearing loss in childhood
| No. | Perinatal | |
|---|---|---|
| 1 | Family historya) of early, progressive, or delayed-onset permanent childhood hearing loss | |
| 2 | Neonatal intensive care of more than 5 days | |
| 3 | Hyperbilirubinemia with exchange transfusion regardless of length of stay | |
| 4 | Aminoglycoside administration for more than 5 daysb) | |
| 5 | Asphyxia or Hypoxic Ischemic Encephalopathy | |
| 6 | Extracorporeal membrane oxygenationa) | |
| 7 | In utero infections, such as herpes, rubella, syphilis, and toxoplasmosis | |
| In utero infection with cytomegalovirusa) | ||
| Mother + Zika and infant with no laboratory evidence & no clinical findings | ||
| Mother + Zika and Infant with laboratory evidence of Zika + clinical findings | ||
| Mother + Zika and Infant with laboratory evidence of Zika - clinical findings | ||
| 8 | Certain birth conditions or findings: | |
| Craniofacial malformations including microtia/atresia, ear dysplasia, oral facial clefting, white forelock, and microphthalmia | ||
| Congenital microcephly, congenital or acquired hydrocephalus | ||
| Temporal bone abnormalities | ||
| 9 | Over 400 syndromes have been identified with atypical hearing thresholdsc). For more information, visit the Hereditary Hearing Loss website [43] | |
| Perinatal or Postnatal | ||
| 10 | Culture-positive infections associated with sensorineural hearing lossc), including confirmed bacterial and viral (especially herpes viruses and varicella) meningitis or encephalitis | |
| 11 | Events associated with hearing loss: | |
| Significant head trauma especially basal skull/temporal bone fractures | ||
| Chemotherapy | ||
| 12 | Caregiver concernd) regarding hearing, speech, language, developmental delay and or developmental regression | |
c) Syndromes [43].
References
1. Yoshinaga-Itano C, Sedey AL, Coulter DK, Mehl AL. Language of earlyand later-identified children with hearing loss. Pediatrics 1998;102:1161-71.



2. Mohr PE, Feldman JJ, Dunbar JL, McConkey-Robbins A, Niparko JK, Rittenhouse RK, et al. The societal costs of severe to profound hearing loss in the United States. Int J Technol Assess Health Care 2000;16:1120-35.


3. American Academy of Pediatrics; Joint Committee on Infant Hearing. Year 2007 position statement: principles and guidelines for early hearing detection and intervention programs. Pediatrics 2007;120:898-921.



4. The Joint Committee on Infant Hearing. Year 2019 position statement: principles and guidelines for early hearing detection and intervention programs. J Early Hear Detect Interv 2019;4:1-44.
5. Thompson DC, McPhillips H, Davis RL, Lieu TL, Homer CJ, Helfand M. Universal newborn hearing screening: summary of evidence. JAMA 2001;286:2000-10.


6. Yoshinaga-Itano C, Gravel JS. The evidence for universal newborn hearing screening. Am J Audiol 2001;10:62-4.


7. Morton CC, Nance WE. Newborn hearing screening--a silent revolution. N Engl J Med 2006;354:2151-64.


8. Chung YS, Oh SH, Park SK. Results of a government-supported newborn hearing screening pilot project in the 17 cities and provinces from 2014 to 2018 in Korea. J Korean Med Sci 2020;35:e251.




9. Park SK, Chang J, Im GJ, Ahn JH, Lee JH, do Han K, et al. Status of early hearing detection and intervention in South Korea: a nationwide population-based study of national infant health checkup. Sci Rep 2020;10:16838.




10. Choi KY, Park SK, Choi S, Chang J. Analysis of newborn hearing screening results in South Korea after National Health Insurance coverage: a nationwide population-based study. Int J Environ Res Pub Health 2022;19:15052.



11. Wroblewska-Seniuk K, Greczka G, Dabrowski P, Szyfter-Harris J, Mazela J. Hearing impairment in premature newborns-analysis based on the national hearing screening database in Poland. PLoS One 2017;12:e0184359.



12. Frezza S, Catenazzi P, Gallus R, Gallini F, Fioretti M, Anzivino R, et al. Hearing loss in very preterm infants: should we wait or treat? Acta Otorhinolaryngol Ital 2019;39:257-62.



13. van Dommelen P, Verkerk PH, van Straaten HL, Dutch Neonatal Intensive Care Unit Neonatal Hearing Screening Working G. Hearing loss by week of gestation and birth weight in very preterm neonates. J Pediatr 2015;166:840-3.e1.


14. Stadio AD, Molini E, Gambacorta V, Giommetti G, Volpe AD, Ralli M, et al. Sensorineural hearing loss in newborns hospitalized in neonatal intensive care unit: an observational study. Int Tinnitus J 2019;23:31-6.


15. Pourarian S, Khademi B, Pishva N, Jamali A. Prevalence of hearing loss in newborns admitted to neonatal intensive care unit. Iran J Otorhinolaryngol 2012;24:129-34.


16. Li PC, Chen WI, Huang CM, Liu CJ, Chang HW, Lin HC. Comparison of newborn hearing screening in well-baby nursery and NICU: a study applied to reduce referral rate in NICU. PLoS One 2016;11:e0152028.



17. Colella-Santos MF, Hein TA, de Souza GL, do Amaral MI, Casali RL. Newborn hearing screening and early diagnostic in the NICU. Biomed Res Int 2014;2014:845308.




18. Chang J, Oh SH, Park SK. Comparison of newborn hearing screening results between well babies and neonates admitted to the neonatal intensive care unit for more than 5 days: analysis based on the national database in Korea for 9 years. PLoS One 2020;15:e0235019.



19. Korver AM, Smith RJ, Van Camp G, Schleiss MR, Bitner-Glindzicz MA, Lustig LR, et al. Congenital hearing loss. Nat Rev Dis Primers 2017;3:16094.




20. Fortnum HM, Summerfield AQ, Marshall DH, Davis AC, Bamford JM. Prevalence of permanent childhood hearing impairment in the United Kingdom and implications for universal neonatal hearing screening: questionnaire based ascertainment study. BMJ 2001;323:536-40.



21. Prieve BA, Schooling T, Venediktov R, Franceschini N. An evidence-based systematic review on the diagnostic accuracy of hearing screening instruments for preschool- and school-age children. Am J Audiol 2015;24:250-67.


22. Shearer AE, Hildebrand MS, Smith RJH. Hereditary hearing loss and deafness overview. In: Adam MP, Everman DB, Mirzaa GM, Pagon RA, Wallace SE, Bean LJH, , editors. GeneReviews(®). Seattle (WA): University of Washington, 2017.
23. Shave S, Botti C, Kwong K. Congenital sensorineural hearing loss. Pediatr Clin North Am 2022;69:221-34.


24. Ko H, Dehority W, Maxwell JR. The impact of maternal infection on the neonate. In: Verma RP, editor. Congenital anomalies in newborn infants - clinical and etiopathological perspectives. London: IntechOpen Ltd., 2021:41.
25. Kim BJ, Han JJ, Shin SH, Kim HS, Yang HR, Choi EH, et al. Characterization of detailed audiological features of cytomegalovirus infection: a composite cohort study from groups with distinct demographics. Biomed Res Int 2018;2018:7087586.




26. Chu CW, Chen YJ, Lee YH, Jaung SJ, Lee FP, Huang HM. Governmentfunded universal newborn hearing screening and genetic analyses of deafness predisposing genes in Taiwan. Int J Pediatr Otorhinolaryngol 2015;79:584-90.


27. D'Aguillo C, Bressler S, Yan D, Mittal R, Fifer R, Blanton SH, et al. Genetic screening as an adjunct to universal newborn hearing screening: literature review and implications for non-congenital pre-lingual hearing loss. Int J Audiol 2019;58:834-50.



29. Gettelfinger JD, Dahl JP. Syndromic hearing loss: a brief review of common presentations and genetics. J Pediatr Genet 2018;7:1-8.



30. Kelsell DP, Dunlop J, Stevens HP, Lench NJ, Liang JN, Parry G, et al. Connexin 26 mutations in hereditary non-syndromic sensorineural deafness. Nature 1997;387:80-3.



31. Kikuchi T, Adams JC, Paul DL, Kimura RS. Gap junction systems in the rat vestibular labyrinth: immunohistochemical and ultrastructural analysis. Acta Otolaryngol 1994;114:520-8.


32. Coyle B, Coffey R, Armour JA, Gausden E, Hochberg Z, Grossman A, et al. Pendred syndrome (goitre and sensorineural hearing loss) maps to chromosome 7 in the region containing the nonsyndromic deafness gene DFNB4. Nat Genet 1996;12:421-3.



33. Reardon W, Coffey R, Phelps PD, Luxon LM, Stephens D, KendallTaylor P, et al. Pendred syndrome--100 years of underascertainment? QJM 1997;90:443-7.


35. Ptok M. Early detection of hearing impairment in newborns and infants. Dtsch Arztebl Int 2011;108:426-31.



37. The Korean Audiological Society. Newborn hearing screening guideline update in Korea. Seoul (Korea): Korean Audiological Society, 2018.
38. Busse AML, Mackey AR, Carr G, Hoeve HLJ, Uhlen IM, Goedegebure A, et al. Assessment of hearing screening programmes across 47 countries or regions III: provision of childhood hearing screening after the newborn period. Int J Audiol 2021;60:841-8.


39. Neumann K, Euler HA, Chadha S, White KR. A survey on the global status of newborn and infant hearing screening. J Early Hear Detect Interv 2020;5:63-84.
40. Neumann K, Mathmann P, Chadha S, Euler HA, White KR. Newborn hearing screening benefits children, but global disparities persist. J Clin Med 2022;11:271.



41. Suh MW, Cho EK, Kim BJ, Chang SO, Kim CS, Oh SH. Long term outcomes of early cochlear implantation in Korea. Clin Exp Otorhinolaryngol 2009;2:120-5.



42. Kim BJ, Kim J, Park IY, Jung JY, Suh MW, Oh SH. Effects of transient auditory deprivation during critical periods on the development of auditory temporal processing. Int J Pediatr Otorhinolaryngol 2018;104:66-71.


43. Van Camp G, Smith R. Hereditary hearing loss homepage [Internet]. Hereditary hearing loss homepage; 2017 [cited 2022 Dec 19]. Available from: https://hereditaryhearingloss.org.






 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link PubMed
PubMed Download Citation
Download Citation


