Article Contents
| Clin Exp Pediatr > Volume 68(1); 2025 |
|
Abstract
Methods
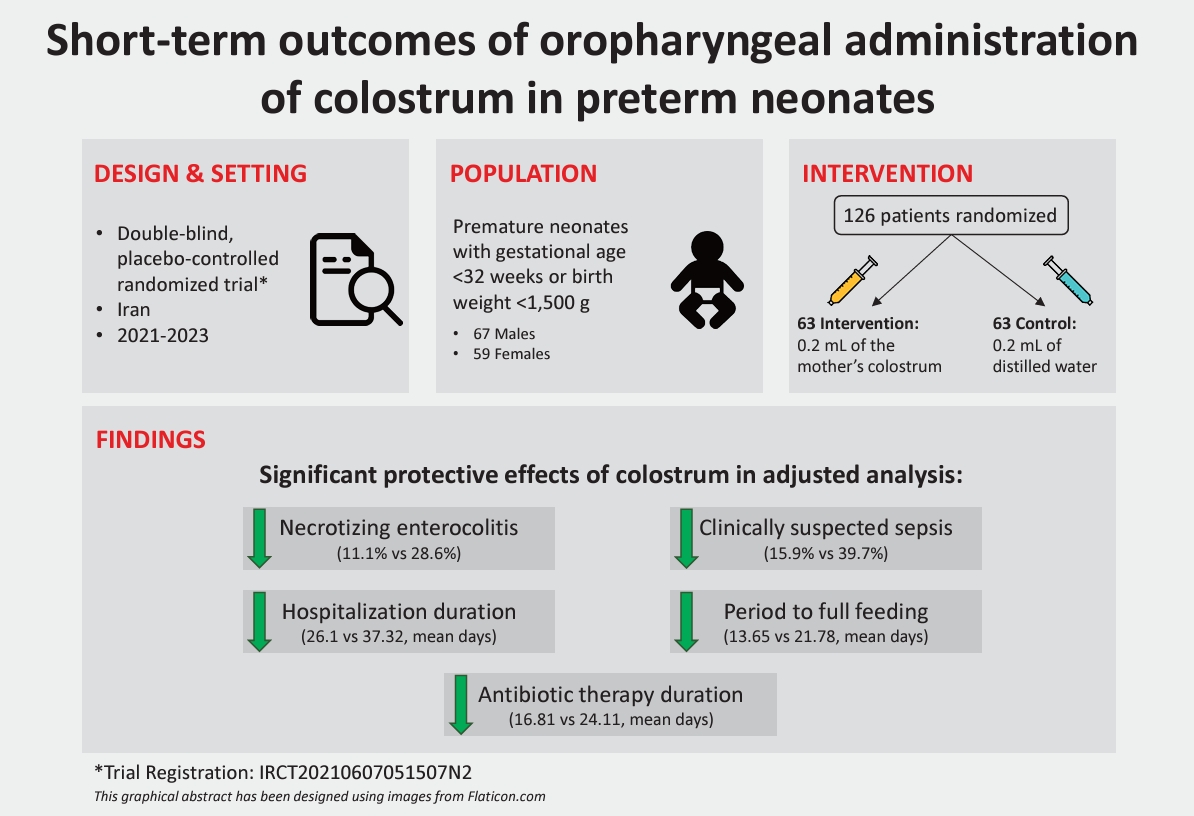
We performed this 2-arm, double-blind, placebo-controlled randomized trial at a tertiary neonatal center in Iran in 2021–2023. The intervention and control arms received 0.2 mL of their mother’s colostrum or distilled water via oropharyngeal administration every 6 hours for 3 days starting from birth until 72 hours of age. The main study outcomes were neonatal death, the incidence of necrotizing enterocolitis, sepsis, retinopathy of prematurity (ROP), length of hospital stay, and period to full enteral feeding. A regression analysis was used to adjust for possible confounders.
Results
A total of 126 neonates (mean gestational age, 30.05 weeks) were randomized to the intervention and placebo groups (n=63 each) and had a mean±standard deviation weight of 1,247±193 g versus 1,156±215 g (P=0.013) and 1- and 5-min Apgar scores of 6.35 versus 5.38 (P=0.003) and 7.84 versus 7.13 (P=0.001), respectively. The mortality rate was 12.7% in the intervention group versus 14.3% in the placebo group (P=0.794). The necrotizing enterocolitis rate was significantly lower in the intervention versus placebo arm (11.1% vs. 28.6%, respectively, P=0.010), as was the clinically suspected sepsis rate (15.9% vs. 39.7%, respectively, P=0.004). The ROP and bronchopulmonary dysplasia rates did not differ significantly between groups after the adjustment for confounders. The mean length of hospital stay was shorter in the intervention group (26.1 days vs. 37.32 days, P=0.023). Moreover, the mean duration of antibiotic therapy and period to full feeding were significantly shorter in the intervention group.
Graphical abstract.
During the past decade the global burden of preterm birth defined as birth before 37 weeks of gestational age and low birth weight (less than 2,500 g), has been alarming and imposed a heavy toll on maternal and child health [1]. The estimated number of preterm births in the year 2020 was about 13.4 million neonates accounting for one of ten births to be too soon around the world [2]. The complications of preterm birth remain the leading cause of under-5 child mortality with about 900,000 deaths globally in 2019 [3]. It is of high importance that these statistics were almost stable in the past decade and no obvious improvements have been achieved [2]. In this regard, the Sustainable Development Goal (SDG) number 3 (target 3.2) proposed to eliminate all preventable neonatal and under-5 deaths by 2030 [4]. SDG number 3 is defined for health by the United Nations and target 3.2 is dedicated to every country to decrease the neonatal mortality rate to 12 or fewer births per every 1,000 live births by 2030 [5].
The etiology of mortality in premature neonates varies based on the gestational age at the time of birth and includes a range of serious conditions like respiratory distress syndrome, necrotizing enterocolitis (NEC), intraventricular hemorrhage (IVH), infection, sepsis, asphyxia, poor nutrition, and temperature dysregulation [6]. Colostrum is the first milk secreted from a mother’s breasts after childbirth and has shown several benefits for neonates especially the premature ones through amplifying the immune system as it is a rich source of immunoglobulin A, lactoferrin, anti-inflammatory cytokines, and growth factors [7,8]. Studies suggest that oropharyngeal administration of colostrum (OAC) might reduce morbidity and mortality in premature neonates by improving the function of the immune system, preventing preterm complications like NEC, and facilitating the start of full enteral feeding [9].
Several interventional studies including randomized controlled trials (RCTs) have tried to investigate the effect of OAC in premature neonates and assess the outcomes; however, robust reviews and meta-analyses of the published literature show most of these trials do not have acceptable sample sizes and their quality is very low, making precise information on the impacts of OAC difficult [10]. Also, the results of some trials have been controversial and the investigators could not reach solid findings on the benefits of OAC in immune markers of premature neonates such as secretory immunoglobulin A and Lactoferrin but they found out that the OAC group reached full-fed state sooner [11]. Besides, most existing RCTs on the health benefits of OAC have primarily investigated the immune-mediated effects of this treatment [8,12-14], and clinical studies exploring more readily observable clinical outcomes are currently limited. As an important factor, most of the mentioned RCTs are conducted in developed and high-income countries, while the burden of preterm birth surges in developing and low and middle-income areas of the world [2]. Therefore, in this study, we aimed to design and conduct RCT to investigate the short-term outcomes and benefits of OAC in Iran.
We designed a 2arm, double-blind, placebo-controlled, single-center randomized trial, conducted in Fatemieh Hospital, a tertiary neonatal center affiliated with Hamadan University of Medical Sciences, Hamadan, Iran. Patient recruitment and randomization happened between October 2, 2021, to March 19, 2023. The end date of the follow-up was June 21, 2023.
This trial was designed and conducted in accordance with the Good Clinical Practice guidelines and the Declaration of Helsinki. The study protocol was reviewed and approved by the ethical committee of Hamadan University of Medical Sciences (code: IR.UMSHA.REC. 1400.446) and registered and approved by the Iranian Registry of Clinical Trials (code: IRCT20210607051507N2) prior to starting the patient recruitment. The recruitment start date was October 2, 2021 Enrolment and participation in this trial was voluntary and all parents of the enrolled neonates provided informed consent before enrolment. The authors did not have access to information that could identify individual participants during or after data collection.
The inclusion criteria for this study were premature neonates with gestational age below 32 weeks at birth or birth weight less than 1,500 g admitted to the neonatal intensive care unit of Fatemieh Hospital even if they required invasive mechanical ventilation. Exclusion criteria were detected neonates with genetic and chromosomal disorders, drug abuser mothers, and those with a positive history of human immunodeficiency virus infection and acquired immunodeficiency syndrome or any condition having contraindication for breastfeeding. This trial was designed to be conducted with a sample size of 126 neonates randomized into study arms. The sample size for this trial was calculated based on the results of a similar RCT conducted before [15], and according to the mean±standard deviation (SD) of the duration of hospitalization outcome in the intervention (37.2±6.7) and placebo (41.5±5.7) arms of the trial, and type II error (α) of 0.05 and type II error (β) of 0.1 (power=90%) by the formula (equation 1) provided below (n=number of samples in each arm, μ=mean, σ=SD), which resulted in 57 samples in each arm which was increased to 63 by considering the 10% rate of patient lost to follow-up.
The study participants were randomized into the 2 study arms of intervention with OAC or placebo with the method of shuffling a deck of cards named A or B held in covered envelopes and in blocks of 2 (AA, AB, BA, BB), prepared by one of the study investigators who was not involved in the process of administration of the agents or patient contact. The final sample was randomly allocated into the 2 study arms with an equal size of 63 neonates—all mothers of the included sample provided colostrum after childbirth. The intervention arm received 0.2 mL of their mother’s colostrum via oropharyngeal administration every 6 hours for 3 days (12 doses) starting from birth to 72 hours of the baby's life. The initial dose was given within 6 hours after admission and stabilization of neonates. The control group received 0.2 mL of distilled water in the same manner. The syringes containing agents were covered by white paper to keep the agent concealed. Because of the low volume of colostrum (0.8 mL per day), we did not have issues with preparing enough colostrum from mothers, so our babies could receive all 12 doses during 3 days of intervention.
Patients were followed up at least 1 month after birth or longer if the time of hospital discharge lasted more. The main outcomes of interest recorded and assessed in this study were the death of neonates, the incidence of NEC stage ≥2 (based on the Bell score [16], and clinical and radiographic findings [ileus, dilated intestinal loops, focal or widespread pneumatosis intestinalis], which represented all in cases in this study to be in stage IIA and ≥B treated by conservative antibiotic regimens and none of them needed surgical treatment), ventilator-associated pneumonia (VAP), IVH, late-onset clinically suspected sepsis, sepsis confirmed by blood culture (B/C), and retinopathy of prematurity (ROP) needing surgical treatment with laser, bronchopulmonary dysplasia (BPD) based on the National Institutes of Health consensus definitions in 2001 which define BPD as a requirement of oxygen supplement for more than 28 days and classify them as mild, moderate, or severe [17]. We included all newborns with BPD in this study without separating them into different groups of mild, moderate, or severe.
Other study variables included the neonate’s sex, weight (g), Apgar scores 1 and 5, type of delivery (normal vaginal delivery [NVD] or Cesarean section), gestational age at the time of birth (week), hospitalization period (day), full enteral feeding period (day), and a number of days the neonate needed antibiotic therapy.
The outcomes of this trial were analyzed based on the intention-to-treat approach and according to a pre-prepared statistical analysis plan. Categorical variables were summarized in frequency and percentage. Quantitative variables were summarized in mean and SD. Pearson chi-square test was used to test the associations between categorical variables and where the sample size did not reach the test’s criteria, Fisher exact test was applied. Independent samples t test was used to compare the means between groups regarding the quantitative variables. We incorporated binary logistic regression (for binary outcomes) and linear regression (for continuous outcomes) models to adjust for the effect of the significantly different variables between trial arms to compare the outcomes regardless of the effect of the potential confounders. Two-tailed P values<0.05 were set as the level of statistical significance. The data were analyzed using IBM SPSS Statistics ver. 22.0 (IBM Co., Armonk, NY, USA).
A total number of 126 neonates including 67 males (53.2 %) and 59 females (46.8%), were randomized in 2 arms of intervention and placebo each one with 63 cases included in the final analysis. Twenty-four neonates were born by NVD and 102 by C-section (P=0.999). The mean±SD weight of patients was higher among the intervention arm compared to the placebo arm (1,247±193 vs. 1,156±215, P=0.013). The mean gestational age of neonates at the time of birth was 30.05 weeks with similar distribution among the 2 arms of study. The evaluated Apgar scores at 1 and 5 minutes were significantly higher among the patients of the intervention group (mean±SD: 6.35±1.64 vs. 5.38±1.89, P=0.003 and 7.84±1.07 vs. 7.13±1.37, P=0.001, respectively). The baseline characteristics of the 2 arms of this trial are summarized in Table 1.
Among the main outcomes evaluated in this study, 12.7% of the intervention group died, compared to 14.3% in the placebo group (P=0.794). The rate of NEC was statistically lower in the intervention arm at 11.1% versus 28.6% (P=0.014), as well as ROP (14.3% vs. 34.9%, P=0.007), BPD (7.9% vs. 25.4%, P=0.009), and clinically suspected sepsis (15.9% vs. 39.7%, P=0.003). The length of hospitalization stay was remarkably lower in the intervention group with a mean of 26.1±18.88 compared to the placebo group with a mean hospital stay of 37.32±19.51 (P=0.001). Also, antibiotic therapy and the period to full feeding were noticeably shorter in the intervention arm (P<0.001).
The rate of IVH and VAP were not significantly different between the 2 arms (P=0.496 IVH, P=0.619 VAP). The rate of sepsis confirmed by B/C was similar between the intervention and placebo group (3.2% in both arms, P=0.999).
After adjusting for statistically significant different factors between trial arms, the differences in outcomes of ROP and BPD were no further significant (P=0.069 and P=0.67); however, the results for the rest of the outcomes remained similar (Table 2).
In this study, we reported the results of an RCT after the administration of oropharyngeal colostrum in premature neonates in a developing country. The main finding of this study was the significantly lower rates of NEC, clinically suspected sepsis, shorter hospital stay, period to full enteral feeding, and antibiotic therapy period in the intervention group with OAC compared to the placebo arm. Although the mortality rates 1 month after birth did not differ significantly between the study arms, noticeable differences in major complications of preterm birth like NEC, highlight the efficacy of OAC via an RCT study.
While our study did not detect a significant difference in all-cause mortality between groups, these findings align with some previous analyses about the impact of OAC in the prevention of mortality before discharge in premature neonates [10,18,19]. However, a recent, larger meta-analysis (n=1,076) reported a significant reduction in mortality rates among the intervention group of OAC compared to the placebo arms (relative risk [RR], 0.71; 95% confidence interval [CI], 0.53–0.94; P=0.02) that are a remarkable finding and highlight the potential benefit of OAC in preventing death in premature infants [20], warranting further investigation with larger trials
The significantly lower rates of NEC in the intervention arm compared to the placebo arm was one of the main findings of this trial. NEC is the most common complication of prematurity affecting the gastrointestinal tract that could be life-threatening [21]. Various methods for NEC prevention in premature neonates have been proposed and OAC is one of them with promising results [22]. A meta-analysis of 4 RCTs on the effect of OAC in the prevention of NEC among very low birth weight neonates found no statistically significant effect of OAC on the incidence of NEC stage ≥2 (RR, 0.64; 95% CI, 0.27–1.49) [23]. Also, other older systematic reviews and meta analyses of RCTs reported no significant protective effect of OAC on NEC [10,19]. In contrast, a later published meta analysis on 11 studies including data from 1,173 patients reported significant protection of OAC on NEC (RR, 0.51; 95% CI, 0.31–0.84; P=0.009) [18]. Also, a recently published meta-analysis on the results of 16 RCTs and a total number of 1,736 patients, reported a significantly lower incidence of NEC stage≥2 according to Bell staging in the intervention group with OAC (RR, 0.56; 95% CI, 0.38–0.84; P=0.004),20) which is in similar to what our study found. Based on the recent pooled results of RCTs, we can conclude our finding is consistent with the body of evidence and OAC could effectively prevent NEC in premature neonates.
As another main finding of the current study, OAC could reduce the rates of ROP in the intervention arm, significantly; however, the results were not further significant in the regression analysis. Some RCTs in the field could not detect noticeable effects of OAC on the prevention of ROP needing surgery, like an RCT on OAC in very low birth weight infants (odds ratio [OR], 1.7; 95% CI, 0.4–6.9) [24], a trial on extremely premature neonates born before a gestational age of 28 weeks (P=0.26) [13], a trial on premature infants born before age of 32 weeks (RR, 1.11; 95% CI, 0.59–2.07; P=0.75) [25], a trial on very low birth weight neonates (P=0.67),15) a meta-analysis published in 2018 (RR, 0.98; 95% CI, 0.33–2.94) [10], a meta-analysis of studies published in 2022 (RR, 1.25; 95% CI, 0.82–1.89; P=0.29), and the most recent published meta-analysis of 7 studies including 991 patients (RR, 1.29; 95% CI, 0.91–1.82; P=0.15) [20].
BPD was the other significantly decreased complication of prematurity in the intervention arm with OAC, although the results were not significant by adjusting for the possible confounders by the regression analysis. Similar to our study, BPD was nonsignificantly altered in a trial on very low birth weight neonates (OR, 0.81; 95% CI, 0.4–1.7) [24], an RCT on extremely premature infants (P=0.58) [13], lower among OAC arm in another trial but not statistically significant (RR, 0.69; 95% CI, 0.27–1.75; P=0.43) [25], another RCT on premature neonates with age less than 32 weeks (P=0.5) [26], another OAC RCT (P=0.71) [15], a meta-analysis of 11 RCTs (RR, 0.83; 95% CI, 0.64–1.08; P=0.17) [18], and the recently published meta-analysis on 8 studies including a sample of 1191 patients (RR, 0.90; 95% CI, 0.73–1.11) [20].
The shorter period of hospital stay and the time needed for full enteral feeding was significantly shorter among the patients undergoing OAC intervention in this study. This finding was comparable to the literature and was consistent with most of the prior evidence. Older meta-analysis of RCTs could not find a significant pooled effect of OAC on hospital stay [10,19]; however, a recently published meta-analysis on 11 RCTs reported a significantly lower duration of hospital stay by OAC intervention (mean difference=-10; 95% CI, -11.36 to -8.64; P<0.001) [18]. Earlier time to full enteral feeding in this study was also consistent with several studies [10,18], including the most recent published meta-analysis on 13 RCTs with a total number of 1,521 patients that found a significantly shorter time to full enteral feeding in the intervention arm with OAC (mean difference=-1.22; 95% CI, -1.99 to -0.45; P=0.002) [20]. The evidence implies the positive outcomes of OAC in the duration of hospital stay and earlier full feeding in premature infants.
This study had some limitations. The limited sample size might be the most prominent one. The limited follow-up period of 1 month or time could be the other limitation of this trial. A limited number of the outcomes of prematurity assessed in this study was the other shortcoming of the study. Another limitation of this study was its monocenteric design which might restrict generalization of the reported results. One major limitation of this trial was significant differences in some of the characteristics of the 2 trial arms including the weight and Apgar scores which could impose some bias in the interpretation of the results; however, by incorporating statistical methods of regression analysis we tried to adjust for the effect of such confounders to reach more robust results. The other limitation of our study was that we did not separate types of BPD (mild, moderate, severe) during data gathering and analysis. The National Institude of Child Health and Human Development (NICHD) 2001 definition states that mild BPD does not lead to significant differences in short-term or long-term outcomes when compared to infants without BPD, so it was better to exclude mild cases when analyzing. Despite all these limitations, this study had several strengths as it was the first RCT evaluating the short-term outcomes of OAC in Iran, a developing country facing the huge burden of preterm birth and associated morbidity and mortality. Future studies with greater sample sizes and including further outcomes are suggested to enrich the evidence on this topic.
Additionally, the results of this study might pave the way for further clinical implications of OAC and research in the field, especially in developing countries where the healthcare systems are challenged with a high burden of preterm birth and shortages of resources.
In conclusion, this study highlighted the significantly lower rates of NEC, late-onset clinically suspected sepsis, and shorter hospital stay, period to full enteral feeding, and antibiotic therapy period in the intervention group with OAC compared to the placebo arm. The reported results of this trial may facilitate the expansion of further clinical prescription of OAC in premature infants to successfully prevent complications of prematurity.
Footnotes
Acknowledgments
We appreciate the help of all nurses who made the conduction of this trial possible.
Table 1.
Patients' baseline characteristics
Table 2.
Study outcome measures
| Variable |
Trial arm |
P value |
||
|---|---|---|---|---|
| Intervention (N=63) | Placebo (N=63) | Unadjusted | Adjusteda) | |
| Mortality (1mo) | 0.794 | 0.099 | ||
| Alive | 55 (87.3) | 54 (85.7) | ||
| Expired | 8 (12.7) | 9 (14.3) | 0.014 | 0.010 |
| Necrotizing enterocolitis | ||||
| Negative | 56 (88.9) | 45 (71.4) | ||
| Positive | 7 (11.1) | 18 (28.6) | ||
| Intraventricular hemorrhage | 0.496 | 0.997 | ||
| Negative | 63 (100) | 61 (96.8) | ||
| Positive | 0 (0) | 2 (3.2) | ||
| Retinopathy of prematurity | 0.007 | 0.069 | ||
| Negative | 54 (85.7) | 41 (65.1) | ||
| Positive | 9 (14.3) | 22 (34.9) | ||
| Ventilator-associated pneumonia | 0.619 | 0.635 | ||
| Negative | 62 (98.4) | 60 (95.2) | ||
| Positive | 1 (1.6) | 3 (4.8) | ||
| Bronchopulmonary dysplasia | 0.009 | 0.067 | ||
| Negative | 58 (92.1) | 47 (74.6) | ||
| Positive | 5 (7.9) | 16 (25.4) | ||
| Clinically suspected sepsis | 0.003 | 0.004 | ||
| Negative | 53 (84.1) | 38 (60.3) | ||
| Positive | 10 (15.9) | 25 (39.7) | ||
| Sepsis confirmed by B/C | 0.999 | 0.860 | ||
| Negative | 61 (96.8) | 61 (96.8) | ||
| Positive | 2 (3.2) | 2 (3.2) | ||
| Hospitalization duration (day) | 26.1±18.88 | 37.32±19.51 | 0.001 | 0.023 |
| Period to full feeding (day) | 13.65±11.51 | 21.78±13.17 | <0.001 | 0.001 |
| Antibiotic therapy duration (day) | 16.81±11.58 | 24.11±10.66 | <0.001 | 0.004 |
References
1. Menon R. Preterm birth: a global burden on maternal and child health. Pathog Glob Health 2012;106:139-40.



2. Organization WH. Born too soon: decade of action on preterm birth. Geneva (Switzerland): World Health Organization, 2023.
3. Perin J, Mulick A, Yeung D, Villavicencio F, Lopez G, Strong KL, et al. Global, regional, and national causes of under-5 mortality in 2000 19: an updated systematic analysis with implications for the Sustainable Development Goals. Lancet Child Adolesc Health 2022;6:106-15.



5. Lawn JE, Bhutta ZA, Ezeaka C, Saugstad O. Ending preventable neonatal deaths: multicountry evidence to inform accelerated progress to the sustainable development goal by 2030. Neonatology 2023;120:491-9.




7. Colonetti T, de Carvalho Florencio I, Figueiredo P, Colonetti L, Rodrigues Uggioni ML, da Rosa MI, et al. Colostrum use and the immune system of premature newborns: a systematic review and meta-analysis. J Hum Lact 2022;38:487-500.



8. Martín-Álvarez E, Diaz-Castro J, Peña-Caballero M, Serrano-López L, Moreno-Fernández J, Sánchez-Martínez B, et al. Oropharyngeal colostrum positively modulates the inflammatory response in preterm neonates. Nutrients 2020;12:413.



9. Xavier Ramos MS, Martins CDC, Souza ES, Vieira GO, Gomes-Filho IS, Figueiredo ACMG, et al. Oropharyngeal colostrum immunotherapy and nutrition in preterm newborns: meta-analysis. Rev Saude Publica 2021;55:59.




10. Nasuf AWA, Ojha S, Dorling J. Oropharyngeal colostrum in preventing mortality and morbidity in preterm infants. Cochrane Database Syst Rev 2018;9:CD011921.


11. Rodriguez NA, Groer MW, Zeller JM, Engstrom JL, Fogg L, Du H, et al. A randomized controlled trial of the oropharyngeal administration of mother s colostrum to extremely low birth weight infants in the first days of life. Neonatal Intensive Care 2011;24:31-5.
12. Glass KM, Greecher CP, Doheny KK. Oropharyngeal administration of colostrum increases salivary secretory IgA levels in very low-birth-weight infants. Am J Perinatol 2017;34:1389-95.



13. Lee J, Kim HS, Jung YH, Choi KY, Shin SH, Kim EK, et al. Oropharyngeal colostrum administration in extremely premature infants: an RCT. Pediatrics 2015;135:e357-66.



14. Maffei D, Brewer M, Codipilly C, Weinberger B, Schanler RJ. Early oral colostrum administration in preterm infants. J Perinatol 2020;40:284-7.




15. Sharma D, Kaur A, Farahbakhsh N, Agarwal S. Role of oropharyngeal administration of colostrum in very low birth weight infants for reducing necrotizing enterocolitis: a randomized controlled trial. Am J Perinatol 2020;37:716-21.


16. Walsh MC, Kliegman RM. Necrotizing enterocolitis: treatment based on staging criteria. Pediatr Clin North Am 1986;33:179-201.



18. Huo M, Liu C, Mei H, Zhang Y, Liu C, Song D, et al. Intervention effect of oropharyngeal administration of colostrum in preterm infants: a meta-analysis. Front Pediatr 2022;10:895375.



19. Panchal H, Athalye-Jape G, Patole S. Oropharyngeal colostrum for preterm infants: a systematic review and meta-analysis. Adv Nutr 2019;10:1152-62.




20. Yan FUZ, Huang C, Lei L, Chen LC, Wei LJ, Zhou J, et al. The effect of oropharyngeal colostrum administration on the clinical outcomes of premature infants: a meta-analysis. Int J Nurs Stud 2023;144:104527.


21. Mϋller MJ, Paul T, Seeliger S. Necrotizing enterocolitis in premature infants and newborns. J Neonatal Perinatal Med 2016;9:233-42.


22. Jin YT, Duan Y, Deng XK, Lin J. Prevention of necrotizing enterocolitis in premature infants - an updated review. World J Clin Pediatr 2019;8:23-32.



23. Garg BD, Balasubramanian H, Kabra NS, Bansal A. Effect of oropharyngeal colostrum therapy in the prevention of necrotising enterocolitis among very low birthweight neonates: a meta-analysis of randomised controlled trials. J Hum Nutr Diet 2018;31:612-24.



24. Ferreira DMLM, Oliveira AMM, de Leves DV, de Bem ÉB, Fatureto GG, Navarro NF, et al. Randomized controlled trial of oropharyngeal colostrum administration in very low-birth-weight preterm infants. J Pediatr Gastroenterol Nutr 2019;69:126-30.


25. OuYang X, Yang CY, Xiu WL, Hu YH, Mei SS, Lin Q. Oropharyngeal administration of colostrum for preventing necrotizing enterocolitis and late-onset sepsis in preterm infants with gestational age≤32weeks: a pilot single-center randomized controlled trial. Int Breastfeed J 2021;16:59.




26. Romero-Maldonado S, Soriano-Becerril DM, Garca-May PK, Reyes-Muoz E, Muoz-Ortz EG, Carrera-Muios S, et al. Effect of oropharyngeal administration of colostrum in premature newborns 32 weeks of gestation on the immune response and neonatal morbidity: a double-blind randomized clinical trial. Front Pediatr 2022;10:891491.



-
METRICS

-
- 1 Crossref
- 0 Scopus
- 7,745 View
- 213 Download





 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link PubMed
PubMed Download Citation
Download Citation

