Article Contents
| Clin Exp Pediatr > Volume 68(2); 2025 |
|
Abstract
Sickle cell disease (SCD) is characterized by chronic hemolytic anemia and intermittent vasoocclusive crises. To date, 4 disease-modifying drugs have been approved for the treatment of SCD: hydroxyurea (an S-phase inhibitor), L-glutamine (an amino acid), crizanlizumab (a P-selectin inhibitor), and voxelotor (a hemoglobin S polymerization inhibitor). Preclinical studies suggested that voxelotor effectively treats SCD and sickle cell anemia (SCA). In a phase III trial, voxelotor-treated patients showed significantly elevated hemoglobin levels (>1 g/dL from baseline) compared to placebo-treated patients. The group that received voxelotor also showed a greater decrease in hemolytic markers but a comparable incidence of side effects. Six ongoing clinical trials also sought to ascertain the effectiveness and safety of high-dose voxelotor when administered to children younger than 12 years. Studies assessing their long-term efficacy and safety are needed to fully understand the role of voxelotor in treating SCD/SCA. In this review, we discuss the mechanisms, trials to date, and future treatment directions of voxelotor.
Sickle cell disease (SCD), a common hemoglobinopathy caused by mutations in the gene encoding the hemoglobin (Hb) beta subunit, was first documented by Herrick in 1910 [1]. He recounted a case of "sickled cells" with a strange presentation of the red blood cells (RBCs) in one of his students; however, he was unable to conclude the disease at that time. Sickle cell anemia (SCA), Hb SC disease (HbSC), sickle cell trait, and Hb sickle-beta-thalassemia (beta-thalassemia positivity or negativity) are subcategories of SCD. Vaso-occlusive crises (VOCs), organ damage, and hemolytic anemia are hallmarks of SCA, the most prevalent type of SCD.
This genetic illness affects millions of people worldwide but is most common in India, the Mediterranean region, and sub-Saharan Africa. Nevertheless, it is also found in other regions of America and Europe. Approximately 300,000 babies are born with SCD each year, over 80% in Africa [2]. India is ranked the second most affected country in terms of expected SCD births, but the prevalence of HbSC appears to vary widely across the Indian subcontinent [3,4].
Due to β-globin gene defects, the Hb S (HbS) molecule is prone to changing into inelastic elongated polymers in a deoxygenated environment. When this conversion becomes irreversible and RBCs assume a permanent sickled shape, the risk of hemolysis and VOCs increases. All forms of SCD share the same pathophysiology, resulting in the polymerization of the HbS component. Organ damage and extreme pain crises are encouraged by these cycles [5,6].
Inhibition of the Gardos channel and cation fluxes, which prevents sickle cell dehydration and inevitable cell death, is one therapy used to impede the pathophysiological mechanisms of SCD. On the other hand, gene therapy is a promising treatment for SCD that involves the insertion or deletion of globins to cause mutations in gene-specific targets. However, they are expensive and unaffordable for many [7]. Four Food and Drug Administration (FDA)-approved disease-modifying drugs are currently available for the treatment of SCD: hydroxyurea (HU), L-glutamine, crizanlizumab, and voxelotor. Before 2017, HU (approved in 1998 for adults and 2007 for children) was the only disease-modifying drug available to treat SCD, followed by the approval of L-glutamine in 2017 and crizanlizumab and voxelotor in 2019 [7,8].
HU and L-glutamine are the most widely prescribed treatments for SCD. Robust evidence supports the efficacy of HU in SCD, including a reduction in VOCs (by 44%), mortality, hospitalizations, and other SCD-related complications [9]. HU reduces sickle cell crisis by promoting fetal Hb (HbF) production; however, it does not affect the sickling process [7-9]. L-Glutamine, an essential amino acid, helps transfer nitrogen to RBCs and produces important substrates; moreover, it reduces VOCs but does not affect sickle cells. As oxidative stress is more prevalent in sickled than normal RBCs, L-glutamine helps reduce it by generating nicotinamide adenine dinucleotide, glutathione, and glutamate. However, the exact biochemical mechanism of glutamine in SCD is not yet well understood [10,11]. In a clinical trial of 230 patients with SCD, those who received L-glutamine had fewer hospitalizations and pain crises than those who received placebo [11].
Crizanlizumab (Adakveo) is a first-in-its-class medication, approved by the FDA in 2019 for use in patients with SCA aged 16 years and older who do not respond to HU. Crizanlizumab binds and inhibits P-selectin; thus, preventing RBCs, leukocytes, and platelets from adhering to blood vessel walls. It also prevents the binding of sickled RBCs, platelets, endothelial cells, and leukocytes. The FDA approved the drug in 2019 after the publication of the Trial to Evaluate Cardiovascular and Other Long-term Outcomes with Semaglutide in Subjects with Type 2 Diabetes (SUSTAIN) trial in which crizanlizumab reduced pain crises in patients with SCD (45% fewer than in the placebo group) [12].
At the same time, on November 25, 2019, voxelotor (GBT440) received approval from the FDA to treat SCD patients aged 12 or older after demonstrating promising outcomes in the GBT_HOPE trial [13,14]. In December 2021, the FDA approved voxelotor to treat SCD in children aged 4 years and above. Voxelotor is an oral medication that inhibits HbS polymerization by reversibly binding to the N-terminal valine of the Hb α-chains and modifying its oxygen affinity. It also prolongs the half-life of RBCs, lowers blood viscosity, prevents HbS sickling, improves the affinity between Hb and oxygen, and shows a positive linear relationship between pharmacokinetics (PK) and pharmacodynamics (PD). Due to these potential advantages, it has been included as part of the PRIME (PRIority MEdicines) scheme of the European Medicines Agency, which supports the development of medications that address unmet medical needs [15-18].
This paper discusses the importance of voxelotor and its effect on sickle RBCs. We collected data on the PK, PD, and clinical use of voxelotor for the treatment of SCD and SCA from pertinent preclinical and clinical studies and ongoing clinical trials. To collect this information, we conducted a thorough literature search using the search terms SCD and voxelotor in the PubMed and Google Scholar databases. Ongoing trials were retrieved from the Clinical Trials Registry (https://clinicaltrials.gov/).
Global Blood Therapeutics manufactures voxelotor (Oxbryta). Volexotor was first authorized through an expedited approval process to treat SCD in adults and adolescents 12 years of age and older who were tolerant of the maximum dosage of HU but did not have anemia [13,19]. This approval was based on the clinical benefit measured by Hb, a biological surrogate endpoint, and a considerable decrease in hemolytic markers [20-23].
Voxelotor (formerly GBT440) is frequently used to treat SCD. Voxelotor and the Nterminal valine of the HbS α-chain form a covalent bond, balancing HbS polymerization and enhancing its oxygenbinding affinity. The molecular mass of this medication is 337.4 g/mol, and its chemical formula is C19H19N3O3, while its chemical name is 2-hydroxy-6 ((2-(1-isopropyl-1H-pyrazol-5-yl) pyridine-3-yl) methoxy) benzaldehyde [16].
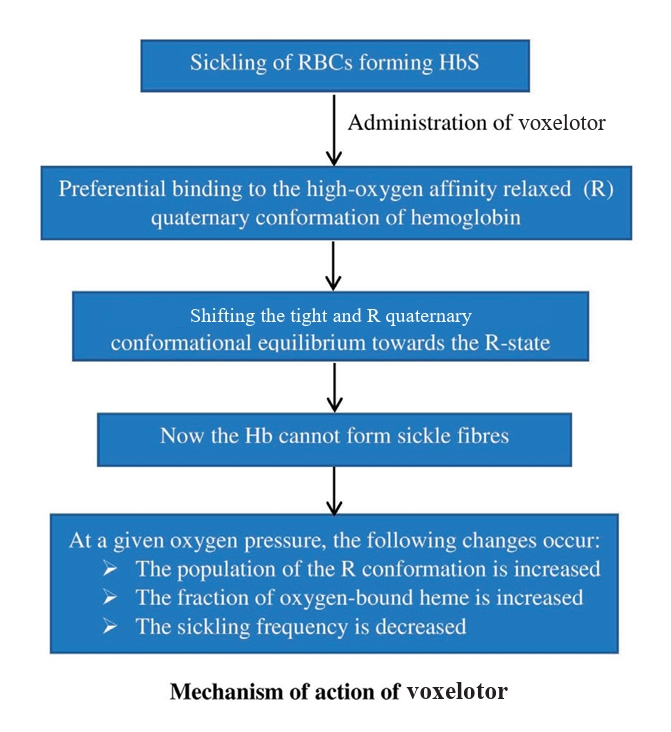
As shown in Fig. 1, voxelotor changes the oxygen affinity of Hb [20]. Tight (T) and relaxed (R) Hb correspond to low and high ligand-binding affinities, respectively. Voxelotor shifts the oxygen affinity of Hb to the left by forming a reversible covalent (Schiff base) bond with the αVal1 nitrogen at the N-terminus of the Hb chain [17]. However, it differs from other compounds because of the hydrogen bond it forms with αSer131 on the alternate α-chain of the same Hb tetramer [21]. The R-state is maintained by a different molecule that ties across the 2 polypeptide chains and binds the alternate αVal1 on the second α-globin chain of the tetramer. This larger molecule forms 1:1 instead of 2:1 stoichiometry because it binds Hb more quickly and effectively, inhibiting HbS polymerization and decreasing serum bilirubin levels [21].
Voxelotor is a well-tolerated drug with a linear PK value, even at high doses. It is metabolized in the liver in 2 phases: oxidation and reduction (phase 1) and glucuronidation (phase 2). It is primarily oxidized in phase 1 by the cytochrome P450 (CYP) enzyme CYP3A4 with some support from CYP2C19, CYP2B6, and CYP2C9. However, while a patient is taking voxelotor, drugs that are metabolized by CYP3A4 (for example, fluconazole) must be avoided because of drug interactions [16,22,23]. The terminal half-life and bioavailability after the oral administration of the voxelotor in patients were 19.1 h and 60%, respectively [21]. A dose-dependent decrease in the partial pressure of O2 (Δp20) occurs when voxelotor binds to Hb and forms a HbS-voxelotor adduct. In a previous study of Δp20, the median percentage of modified Hb on day 28 was 25% in 11 patients with SCA receiving voxelotor 1,000 mg/day [17].
In 2019, Hutchaleelaha et al. [17] used a Hemox Analyzer (TCS Scientific, New Hope, PA, USA) to determine changes in Hb oxygen affinity and oxygen equilibrium curves. After being collected in vacutainers filled with sodium citrate, the samples were examined to determine their Δp20 and Δp50 values. According to the authors, voxelotor caused a dose-dependent expansion in oxygen affinity and modified Δp50.
According to studies conducted by Patel et al. in 2013 [24] and 2014 [25], deoxygenated samples of blood that were incubated with voxelotor had longer RBC half-lives, as the drug was able to decrease hemolysis and whole-blood viscosity and reduce RBC deformability. These findings highlight the significance of HbS polymerization and sickling in compromised RBC functionality and survival in SCD. Blood deoxygenated and treated with voxelotor showed improved viscosity compared to untreated deoxygenated and oxygenated blood. These findings suggest that increasing the oxygen affinity of RBCs helps reduce the amount of time spent by RBCs in the most hypoxic environment and improves its rheology [26].
In 2016, Oksenberg et al. [23] demonstrated that GBT440 (voxelotor) binds particularly to Hb and that the RBC: plasma ratio was 150:1 in various animal species. At 2-hour postadministration, the rates of HbS deoxygenated in vitro without preincubation with voxelotor and stoichiometrically with voxelotor were 76% and 17%, respectively. Oxygen affinity was demonstrated to be dependent on voxelotor concentration. This substantial shift in affinity is expected to prevent HbS polymerization and sickling in hypoxic environments. This was demonstrated by the fact that the presence of 20%–30% modified HbS increased the polymerization delay time from 9 minutes to 18–22 minutes, which was equivalent to the known effect of the same concentration of HbF on delay time [23].
GBT440 also prolonged the half-life of RBCs, reduced the number of reticulocytes, and prevented ex vivo RBC sickling in a murine model of SCD in which mice were given 100–150 mg/kg of the drug through oral gavage for 9–12 days. These findings suggested the possibility of once-daily dosing of GBT440 for individuals with high therapeutic safety [23].
In 2018, Dufu et al. [16] examined the mechanical and rheological properties of GBT440-treated sickle RBCs (SS-RBCs). They found that GBT440 treatment retained the SSRBCs’ deformability and improved blood viscosity by preventing HbS polymerization under deoxygenated conditions. They also found voxelotor-modified HbS as helpful as HbF since it defers HbS polymerization, supporting the development of GBT440 as a disease-modifying drug for patients with SCD.
In vitro tests using blood samples from patients with SCD and in vivo animal studies supported the possibility of once-daily treatment with voxelotor, which has a suitable half-life and is highly specific to Hb [16]. Additionally, voxelotor lowers blood viscosity, lengthens RBC half-life, decreases HbS sickling, eliminates sickled RBC deformities, and has a good linear PK-PD relationship [16,17,23].
In a double-blind randomized controlled trial (RCT), the safety and efficacy of voxelotor was compared between 40 healthy volunteers and 8 patients with SCD. The healthy volunteers were randomized and administered a single dose of voxelotor in ascending doses of 100, 400, 1,000, 2,000, or 2,800 mg or placebo, while the 8 patients with SCD were administered voxelotor 1,000 mg/day [17]. With a half-life range of 61±7 to 85±7 hours and a linear PK profile, voxelotor was easily tolerated. A 38.9% Hb modification was noted after 15 days of treatment with 900 mg of voxelotor. The terminal half-life of voxelotor (50±3 hours) was shorter in patients with SCD than in healthy persons. The results of erythropoietin (EPO) testing, exercise testing, and hematological parameters indicated normal oxygen delivery at rest and during exercise. A change in the affinity of Hb for oxygen is linked to its PK, resulting in a therapeutic window of 20%–30% [17].
The phase 3 double-blind HOPE RCT compared the safety and efficacy of 2 doses of voxelotor in 274 patients aged 12–65 years. One group was randomly chosen to receive a placebo, while the other group was given 900 or 1,500 mg of voxelotor once daily [13]. Compared with patients in the placebo group, 51.1% of those receiving the higher voxelotor dose had significantly higher Hb levels after 24 weeks of treatment. The participants in the placebo (26%) and voxelotor (23%) groups experienced the same rate of adverse drug reactions (26% in patients taking 1,500 mg vs. 23% in those taking 900 mg) [13]. The important clinical studies are summarized in Table 1.
Howard et al. [27] conducted a phase 1/2 study of healthy volunteers and patients with SCD to evaluate the PK and PD properties, safety, and tolerability of voxelotor by assessing the clinical measures of anemia, hemolysis events, and the percentage of sickled RBCs. For 28 days, voxelotor (500, 700, or 1,000 mg) or placebo was administered once daily to 38 patients with SCD. Sixteen patients received either 700 or 900 mg of voxelotor or placebo for 90 days. After 90 days, 4 patients from the group were included in a 6-month extension study in which they received daily treatment with 900 mg of oral voxelotor. Every individual who used voxelotor for more than 28 days at any dose exhibited notable hematological improvements, such as elevated Hb levels, decreased hemolysis, and fewer sickled RBCs. There was no evidence of tissue hypoxia during exercise or adverse effects on oxygen uptake threshold, RBC count, or heart rate at normal or intense treatment levels [27].
In a longitudinal follow-up study of HOPE trial patients, Howard et al. [28] reported significant improvement in Hb concentration and decreased VOCs, pain crises, and hemolytic markers (bilirubin and reticulocytes) from baseline to 72 weeks in the voxelotor 1,500 mg versus placebo group. They demonstrated that an increase in Hb level was inversely linked to a decrease in VOC occurrences. Seven patients aged 22–67 years were treated with 900 mg of voxelotor once daily with the possibility of increasing the dose to 1,500 mg for 6–17 months according to a case series published by Blyden et al. [29] Except for diarrhea in 2 patients taking 1,500 mg of voxelotor daily, there have been no reports of voxelotor side effects. During treatment, 2 patients with organ damage died, but this was unrelated to voxelotor therapy.
In 2020, Herity et al. [20] examined the pharmacological properties, clinical data, and use of voxelotor to treat patients with SCD using data from 3 clinical trials, case reports, and series (phase 1). The authors identified 3 drugs authorized for managing SCD and related complications; however, these drugs did not specifically improve the underlying condition. According to the authors, the novel drug voxelotor targeted the pathophysiology of SCD and was generally well-tolerated.
The four-part, phase 2a, open-label, single- and multiple-dose HOPE-KIDS-1 trial conducted by Estepp et al. [30] is currently underway to evaluate the PK, efficacy, and safety of voxelotor, has released its preliminary findings. Forty-five patients aged 4–11 years with SCD were included in the study cohort and treated with voxelotor for up to 48 weeks. Of them, 34 had their Hb response efficacy endpoint defined as an increase in Hb of >1 mg/dL from baseline to 24 weeks. The findings revealed that 47% (n=16 of 34) of the cases had a Hb response (>1 mg/dL increase) at week 24, while 35% (12 of 34) and 21% (7 of 34) of the cases had Hb increases of >1.5 and >2.0 g/dL from baseline, respectively [30].
Muschick et al. [31] retrospectively assessed the hematologic response to voxelotor therapy in 77 patients aged 12–70 years (mean age, 30.4 years) with SCD. Of them, 23 (30%) were younger than 21 years of age. Most patients (86%) with the homozygous HbSS genotype also received HU. The duration of voxelotor therapy ranged from 1.9 to 17 months (mean duration, 9.7 months), and 74 patients received a once-daily dose of 1,500 mg voxelotor. All patients showed a favorable response as indicated by a significant increase in Hb levels and decline in reticulocyte percentage and total bilirubin from baseline. More significant improvement was seen with concurrent HU than voxelotor therapy alone, suggesting a complementary effect.
By examining the claims data from patients with SCD in regular clinical practice in 2019–2021, Shah et al. [32] assessed the practical efficacy of voxelotor for the treatment of patients with SCD using a nationwide medical claim dataset (N=3,128). The authors found that 60.8% of patients had an increase in Hb >1 g/dL after starting voxelotor, while those who received more than one transfusion before therapy (n=190) experienced a 52% decrease in mean transfusion rate per patient-year. Decreases in mean VOC frequency (-23%), length of stay (-30%), usage of iron chelation (-46%), and prescribed opioids (-13%) were seen in patients having one of the related events (n=1,065). These results support the hypothesis that voxelotor can lower VOCs and transfusions in clinical settings.
Brown et al. [33] conducted one-time semi-structured interviews of adults, children, and caregivers to learn more about the experiences and perspectives of patients with SCD receiving voxelotor treatment. They discovered that nearly all patients reported improvements in their self-reported health-related quality-of-life and voxelotor-treated SCD symptoms, particularly fatigue, jaundice, and pain crises.
Despite showing favorable hematologic responses and improved rheology in many preclinical and clinical studies, concerns persist regarding the safety of voxelotor [20]. First, the extreme leftward shift in the oxygen dissociation curve could reduce the tissue's oxygen supply. Owing to its strong oxygen affinity to bind oxygen (at high partial pressure) in pulmonary capillaries and its weak oxygen affinity to release oxygen at the lower partial pressure of active tissues, Hb can bind and release oxygen very efficiently [34].
Preclinical data from voxelotor analogs suggest that the p50 for altered Hb would be shifted as far as, or even more than, that of HbF during drug development. Therefore, some have questioned whether a targeted 30% modification could further reduce oxygen delivery in a patient population already experiencing reduced tissue oxygen delivery due to severe anemia by another 30%, particularly in the context of exertion or acute respiratory failure. However, a true drop in the functional oxygen content of this magnitude could significantly increase the risk of stroke in individuals already at high risk of stroke due to sickle cell-related cerebrovascular illness [35-37].
Although p50 showed a significant reduction (>80%) in voxelotor-modified HbS, this remains a valid concern. Many researchers have recognized this concern, which has been addressed by including EPO in preclinical studies and clinical trials. Because EPO is a biomarker of oxygen delivery, patients receiving voxelotor treatment may have some degree of reduced oxygenation that could require shunting. All 3 stages of pharmacological development conform from this perspective [34].
Another concern has been raised regarding the potential for increased viscosity due to elevated hematocrit levels. SCD transfusion procedures and the well-known risk of "over-transfusing" to Hb >10 g/dL are the main causes of this problem. To determine the involved risks, data were examined from a subanalysis of the Stroke Prevention Trial in Sickle Cell Anemia 2 (STOP2) trial that focused on the effect of Hb level on the risk of stroke following the cessation of transfusions. However, the trial results contradicted this assertion by showing a negative relationship between Hb and transcranial Doppler (TCD) velocity after the transfusions ceased. Although these results are encouraging, it is difficult to pinpoint the exact cause or degree of Hb increase in patients from the STOP2 trial versus those treated with voxelotor [38].
Phan et al. [39] conducted a recent pilot study examining the impact of voxelotor on exercise capacity in 10 patients with SCA and high HbF levels who were 12 years of age or older and stable on HU therapy. Cardiopulmonary exercise testing (CPET) was used to assess each subject's ability to exercise before and after 8 weeks of daily 1,500 mg voxelotor therapy. The subjects' hematologic parameters were evaluated before each CPET session. While voxelotor therapy improved their hematologic parameters, 9 of the patients showed no improvement in peak oxygen consumption. The authors concluded that their oxygen delivery may have been limited due to an altered oxygen dissociation curve, which might have contributed to the lack of improvement in exercise capacity in the patients with SCA treated with voxelotor.
Regarding the adverse effects of voxelotor, Vichinsky et al. [13] and Hutchaleelaha et al. [17] found that voxelotor was rapidly absorbed and well-tolerated with a similar incidence of adverse drug reactions in both groups. A few other studies reported minor adverse effects, such as headache, diarrhea, abdominal cramps, rash [27], headache and loose stools [28], diarrhea [29], and headache, diarrhea, nausea, and arthralgia [20]. Estepp et al. [30] concluded that voxelotor was well-tolerated even by younger patients (up to 4 years old). Muschick et al. [31] also reported that the adverse effects were infrequent, mild, and self-limiting and disappeared with dose adjustments.
The HOPE trial of voxelotor showed promising results. Voxelotor dispersible pills resulted in rapid and sustained Hb formation as well as reduced hemolysis. The FDA provided review permission to use voxelotor in tablet form. According to an FDA-released official statement, ongoing research on voxelotor can confirm that Hb and oxygen-binding affinity provide a clinical benefit [21]. Nearly 12 ongoing clinical trials have evaluated the tolerability, effectiveness, and pharmacological profile of voxelotor for managing SCD and SCA in adults and children. These important ongoing trials are summarized in Table 2.
To determine how voxelotor affects cerebral hemodynamics in patients aged 4–30 years with SCD and SCA, an open-label single-arm trial called the effect of VoxSCAN (voxelotor on Cerebral Hemodynamic Response in Children with Sickle Cell Anemia) was planned. For 12 weeks, qualified individuals were administered a daily dose of 1,500 mg of voxelotor. Four and 12 weeks later, alterations in cerebral blood flow, oxygen extraction fraction, and cerebral metabolic rate of oxygen were assessed [40].
The Open-Label Extension of Voxelotor is a phase 3 trial enrolling more than 600 participants with SCD at over 70 clinical sites globally. All patients with SCD aged 6 months to 18 years will be recruited and will receive once-daily oral voxelotor. This trial will evaluate the safety of voxelotor and complications related to SCD in patients receiving long-term voxelotor [41].
Another open-label extension study, named 034OLE (GBT440-031), aimed to evaluate the long-term safety and therapeutic effects of voxelotor. Approximately 100 clinical sites are participating, and 435 eligible patients from trial GBT440-031 have been recruited. The primary data collection started in June 2018, and the study is scheduled to be completed in October 2024 [42].
The HOPE-Kids-2 trial is a phase 3, double-blind RCT that will assess how voxelotor affects TCD readings in children with SCD. A total of 224 children with SCD aged 2–15 years were recruited and randomly assigned in a 1:1 ratio to receive either placebo or voxelotor. The main outcome measure is the change in time-averaged maximum mean velocity arterial cerebral blood flow as determined by TCD at 24 weeks, while the secondary outcome measures are changes in TCD flow velocity and various hemolysis markers from baseline to 24, 48, and 96 weeks [43].
An open-label multicenter study named "Expanded Access Protocol for Pediatric Patients with Sickle Cell Disease Who Have No Alternative Treatment Options" is planned to provide early access to voxelotor therapy among pediatric patients with SCD aged 6 months to 11 years before market authorization. Oral voxelotor will be administered once daily at a weight-based dosage to children who are ineligible to participate in voxelotor trials and have no other available treatment options. The management of the participants will follow standard protocols for SCD, and regular clinical and laboratory assessments will be performed every 12 weeks during routine visits. A safety follow-up appointment is scheduled for 28±7 days following the last voxelotor dose [44].
The recently approved HbS polymerization inhibitor voxelotor has the potential to treat SCD in adults and children aged 4 years and older. According to published data, voxelotor can be used as a disease-modifying drug to treat SCD. We hope that the results of the ongoing trials will demonstrate its advantageous role in younger children as well. Voxelotor lowers hemolysis-related laboratory markers while increasing Hb levels. Moreover, it works well and is safe when used alone or in conjunction with HU. However, long-term safety and efficacy data are required to fully understand the role of voxelotor in managing SCD.
Table 1.
Important clinical studies of voxelotor use for treating sickle cell disease
| Study | Location | Study design | Sample size | Study population | Inter-vention | Objectives | Results | Side effects |
|---|---|---|---|---|---|---|---|---|
| Vichinsky et al. [13] 2019 | Multicenter | HOPE trial - phase 3 randomized, double-blind, placebo-controlled trial | 274 | Confirmed SCD patients aged 12–65 yr (median: 24 yr) with Hb of 5.5–10.5 g/dL at enrolment, and one and 10 VOCs in the previous 12 mo | Group 1: voxelotor 1,500 mg OD (n=90), group 2: voxelotor 900 mg OD (n=92), group 3: placebo (n=92) | Percentage of patients showing Hb response (>1.0 g/dL increase from baseline at week 24) | The response rate was 51% in group 1 vs. 7% in group 3 (P<0.001). Significant decrease in markers of hemolysis (indirect bilirubin; P<0.001 & reticulocyte count (P<0.001) at week 24. The annual VOCs were 2.77 vs. 2.76 vs. 3.19 | Common side effects (headache, diarrhea, and nausea) were similar (26% in group 1 vs. 23% in group 2 & 26% in group 3) |
| Hutchaleelaha et al. [17] 2019 | USA, UK | Phase 1/2, double-blind, randomized, placebo-controlled trial, 3-part study, single ascending dose design | 40 Healthy volunteers and 8 SCD patients | Healthy volunteers (18–55 yr) old SCD patients (18–60 yr) | Voxelotor (100, 400, 1,000, 2,000, or 2,800 mg) to healthy volunteers and 1,000 mg to SCD patients. Each cohort received voxelotor (6) or placebo (2) | Safety, tolerability, PK, and PD in healthy volunteers and SCD patients | Hb modification with voxelotor (900 mg) was 38%±9%. It showed a linear PK and half-life of 61±7 hr to 85±7 hr. The half-life in SCD (50±3 hr) was shorter than in healthy volunteers. | Well tolerated with no dose-limiting toxicities. |
| Howard et al. [27] 2019 | UK | Phase 1/2, double-blind, randomized, placebo-controlled trial, 3-part study, single ascending dose design | 38 Patients received voxelotor for 28 day and 16 patients for 90 day | SCD patients aged 18–60 yr and weighing >50 kg | Voxelotor multiple doses (500, 700, or 1,000 mg) for 28 day and multiple doses (700 or 900 mg) for 90 day | Safety, tolerability, PK, and PD in SCD patients | Voxelotor therapy for ≥28 day resulted in an increase in Hb and reduction in markers of hemolysis and percentage of sickled red cells | Well tolerated with no treatment-related serious adverse events |
| Howard et al. [28] 2021 | Multicenter, 60 clinical sites | 72-Wk follow-up of HOPE trial | 274 | Confirmed SCD patients aged 12–65 yr (median: 24 yr) with Hb of 5·5-10·5 g/dL at enrolment, and one and 10 VOCs in the previous 12 months | Group 1: voxelotor 1,500 mg OD (n=90), group 2: voxelotor 900 mg OD (n=92), group 3: placebo (n=92) | Long-term efficacy (change in Hb from baseline, markers of hemolysis, VOC events) and safety at 72 wk of voxelotor therapy | 89% of patients in group 1 had an increase in Hb of ≥1 g/dL vs. 25% in group 3 (P<0.001) at week 72. A significant decline in hemolysis markers (indirect bilirubin, reticulocyte, LDH) was seen with a nonsignificant decrease in VOCs | Infrequent (<10%); grade 3 or 4 adverse events . Anemia was seen in 2 patients of group 1, 7 o f group 2, and 3 patients of group 3) |
| Estepp et al. [30] 2021 | Multicenter (15 study sites in the USA, UK & Lebanon) | (HOPE-KIDS 1) | 45 Children with SCD | Pediatric patients with SCD aged 4–11 yr (median 7 yr) with Hb ≤10.5 g/dL at baseline | Voxelotor equivalent to 1,500 mg OD | To evaluate PK, hematological response, and safety | 47% Achieved Hb response (>1 g/dL increase in Hb) | The most common side effects were diarrhea (11%), vomiting (11%), & rash (9 %) . No grade 3 or 4 side effects or deaths were observed. |
| Four-part, phase 2a, open-label, single and multiple-dose trial | Markers of hemolysis were decreased (mean change at week 24: -28.6% indirect bilirubin, -2.6% LDH, & -3.3% reticulocytes). The half-life was 27.2 hr. | |||||||
| Muschick et al. [31] 2022 | USA | Single-center retrspective chart review | 77 | Confirmed SCD patients aged 12–70 yr (mean age, 30.4 yr). 30% were <21 yr of age | Voxelotor 1,500 mg/day or equivalent (mean duration, 9.7 mo; range, 1.9–17 mo) | Hematological response and quality-of-life in SCD patients | All patients showed favorable responses suggested by increased Hb (mean increase of 1.49 g/dL), decreased total bilirubin and reticulocytes, and subjective clinical improvement | 4 Patients reported mild side effects requiring dose modification: diarrhea (n=2), rash (n=1), and fever (n=1) |
| Phan et al. [39] 2023 | USA | Open-label, single-arm, longitudinal interventional pilot study | 10 | Conf irmed SCD patients aged 12–24 yr | Voxelotor 1,500 mg once a day. | Exercise capacity measured by change in peak oxygen consumption (VO2) | All patients showed an Hb rise (mean rise +1.6 g/dL). Changes in peak VO2 ranged from -12.8% to +11.3% and 9 of 10 patients showed no improvement | |
| Cardiopulmonary exercise test before & after voxelotor | ||||||||
| Shah et al. [32] 2022 | USA | Retrospective review of national medical claims database | 3,128 | Confirmed SCD patients aged ≥12 yr (mean, 34.7 yr) who started voxelotor therapy from November 2019 to June 2021 | Effectiveness of voxelotor in SCD patients in clinical practice | 60.8% Showed a>1 g/dL increase in Hb (n=74). The mean transfusion rate/patient-year decreased by 52% in patients requiring ≥1 transfusion (n=190). The mean number of VOCs decreased by 23% in patients with ≥1 VOCs (n=1,065), |
Table 2.
Ongoing trials evaluating voxelotor
| Trial | Country | Status | Study design | Sample size | Study population | Intervention | Objective | Endpoints |
|---|---|---|---|---|---|---|---|---|
| VoxSCAN (NCT05 018728) [39] | United States | Recruiting, estimated primary completion: Dec 2024 | Phase 2, open-label, intervention study | 50 | Confirmed SCD patients aged 4–30 years with Hb ≤10.5 g/dL at baseline | Voxelotor 1,500 mg once a day or weight-adjusted equivalent dose for 12 wk | To assess voxelotor’s effects on cerebral hemodynamics | Primary: change in cerebral blood flow, oxygen extraction fraction, and cerebral metabolic rate of oxygen at 4 and 12 wk |
| Secondary: change in Hb and RBC content at 4 & 12 wk | ||||||||
| Open-Label Extension Study of Voxelotor (NCT041 88509) [40] | Multicenter (25 sites) | Recruiting, estimated primary completion: Mar 2028 | Phase 3, intervention, open-label extension study | 300 | SCD patients aged 6 mo to 18 yr, who received the drug in a voxelotor study | Voxelotor 1,500 mg once a day or equivalent dose | Safety and SCD-related complications of long-term Voxelotor therapy | Primary: treatment-emergent adverse events and serious adverse events; frequency of SCD-related complications |
| Open-Label Extension Study of Voxelotor 034OLE (NCT03573882) [41] | Multicenter (globally 100 sites) | Active, not recruiting, estimated primary completion:Mar2028 | Phase 3, intervention, open-label extension study | 435 | Participants from GBT440-031 who have completed 72 wk of treatment | Voxelotor 900 or 1,500 mg once a day | Long-term safety and effects of voxelotor in participants who have completed treatment in study GBT440-031 | Primary: treatment-related adverse effects; frequency of SCD-related complications |
| Secondary: response in hemolytic anemia | ||||||||
| HOPE-KIDS 2 (NCT04218084) [42] | Multicenter (globally 51 sites) | Active, not recruiting, estimated primary completion: Jan 2025 | Phase 3, randomized, doubleblind placebocontrolled trial | 236 | Confirmed SCD patients aged 2–14 years | Randomized 1:1 to receive voxelotor 1,500 mg (or equivalent) once a day or a matching placebo | Effect of Voxelotor on transcranial Doppler (TCD) flow measurements | Primary: change in TCD measurement (24 wk) |
| Secondary: change in TCD flow velocity (FV), conversion to abnormal FV, reversion to normal FV, TCD FV reduction ≥15 cm/sec, Hb level, and measures of hemolysis | ||||||||
| Expanded access protocol for adults and pediatric patients (NCT04724421) [43] | 37 Locations in the United States and Brazil | Available | Expanded access protocol | All eligible patients | Confirmed SCD patients aged 6 mo to 11 yr | Voxelotor (weight-adjusted dose) | To provide Voxelotor for the treatment of SCD in pediatric patients who have no alternative treatment options | Expanded access to voxelotor |
References
1. Herrick JB. Peculiar elongated and sickle-shaped red blood corpuscles in a case of severe anemia. 1910. Yale J Biol Med 2001;74:179–84.


2. Salinas Cisneros G, Thein SL. Recent advances in the treatment of sickle cell disease. Front Physiol 2020;11:435.


3. Lehmann H, Cutbush M. Sub-division of some Southern Indian communities according to the incidence of sickle-cell trait and blood groups. Trans R Soc Trop Med Hyg 1952;46:380–3.

4. Hockham C, Bhatt S, Colah R, Mukherjee MB, Penman BS, Gupta S, et al. The spatial epidemiology of sickle-cell anaemia in India. Sci Rep 2018;8:17685.




5. Mangla A, Ehsan M, Agarwal N, Maruvada S. Sickle cell anemia. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing, 2024.
6. Sundd P, Gladwin MT, Novelli EM. Pathophysiology of sickle cell disease. Annu Rev Pathol 2019;14:263–92.


7. Shah F, Dwivedi M. Pathophysiology and recent therapeutic insights of sickle cell disease. Ann Hematol 2020;99:925–35.



8. Barak M, Hu C, Matthews A, Fortenberry YM. Current and future therapeutics for treating patients with sickle cell disease. Cells 2024;13:848.



9. Hasson C, Veling L, Rico J, Mhaskar R. The role of hydroxyurea to prevent silent stroke in sickle cell disease: systematic review and meta-analysis. Medicine (Baltimore) 2019;98:e18225.




10. Cox SE, Hart E, Kirkham FJ, Stotesbury H. L-Glutamine in sickle cell disease. Drugs Today (Barc) 2020;56:257–68.


11. Niihara Y, Miller ST, Kanter J, Lanzkron S, Smith WR, Hsu LL, et al. A phase 3 trial of l-glutamine in sickle cell disease. N Engl J Med 2018;379:226–35.


12. Ataga KI, Kutlar A, Kanter J, Liles D, Cancado R, Friedrisch J, et al. Crizanlizumab for the prevention of pain crises in sickle cell disease. N Engl J Med 2017;376:429–39.


13. Vichinsky E, Hoppe CC, Ataga KI, Ware RE, Nduba V, El-Beshlawy A, et al. A phase 3 randomized trial of voxelotor in sickle cell disease. N Engl J Med 2019;381:509–19.


15. Telfer P, Agodoa I, Fox KM, Burke L, Mant T, Jurek M, et al. Impact of voxelotor (GBT440) on unconjugated bilirubin and jaundice in sickle cell disease. Hematol Rep 2018;10:7643.




16. Dufu K, Patel M, Oksenberg D, Cabrales P. GBT440 improves red blood cell deformability and reduces viscosity of sickle cell blood under deoxygenated conditions. Clin Hemorheol Microcirc 2018;70:95–105.



17. Hutchaleelaha A, Patel M, Washington C, Siu V, Allen E, Oksenberg D, et al. Pharmacokinetics and pharmacodynamics of voxelotor (GBT440) in healthy adults and patients with sickle cell disease. Br J Clin Pharmacol 2019;85:1290–302.




18. Han J, Saraf SL, Gordeuk VR. Systematic review of voxelotor: a first-in-class sickle hemoglobin polymerization inhibitor for management of sickle cell disease. Pharmacotherapy 2020;40:525–34.



19. Lehrer-Graiwer J, Yokoshima L, Tong B, Love TW. Accelerated approval of Oxbryta® (voxelotor): a case study on novel endpoint selection in sickle cell disease. Contemp Clin Trials 2020;98:106161.


20. Herity LB, Vaughan DM, Rodriguez LR, Lowe DK. Voxelotor: a novel treatment for sickle cell disease. Ann Pharmacother 2021;55:240–5.



21. Metcalf B, Chuang C, Dufu K, Patel MP, Silva-Garcia A, Johnson C, et al. Discovery of GBT440, an orally bioavailable R-state stabilizer of sickle cell hemoglobin. ACS Med Chem Lett 2017;8:321–6.


22. Torres L, Conran N. Emerging pharmacotherapeutic approaches for the management of sickle cell disease. Expert Opin Pharmacother 2019;20:173–86.


23. Oksenberg D, Dufu K, Patel MP, Chuang C, Li Z, Xu Q, et al. GBT440 increases haemoglobin oxygen affinity, reduces sickling and prolongs RBC half-life in a murine model of sickle cell disease. Br J Haematol 2016;175:141–53.



24. Patel M, Oksenberg D, Silva A, Betz A, Metcalf B, Sinha U. GTx011, a novel agent that improves rheological properties of sickle cell blood by increasing oxygen affinity for hemoglobin. Blood 2013;122:2207.


25. Patel M, Cabrales P, Dufu K, Metcalf B, Sinha U. GTx011, an anti-sickling compound, improves SS blood rheology by reduction of HbS polymerization via allosteric modulation of O2 affinity. Blood 2014;124:1370.


26. Patel MP, Siu V, Silva-Garcia A, Xu Q, Li Z, Oksenberg D. Development and validation of an oxygen dissociation assay, a screening platform for discovering, and characterizing hemoglobinoxygen affinity modifiers. Drug Des Devel Ther 2018;12:1599–607.


27. Howard J, Hemmaway CJ, Telfer P, Layton DM, Porter J, Awogbade M, et al. A phase 1/2 ascending dose study and open-label extension study of voxelotor in patients with sickle cell disease. Blood 2019;133:1865–75.




28. Howard J, Ataga KI, Brown RC, Achebe M, Nduba V, El-Beshlawy A, et al. Voxelotor in adolescents and adults with sickle cell disease (HOPE): long-term follow-up results of an international, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Haematol 2021;8:e323–33.


29. Blyden G, Bridges KR, Bronte L. Case series of patients with severe sickle cell disease treated with voxelotor (GBT440) by compassionate access. Am J Hematol 2018;May 12 doi: 10.1002/ajh.25139. [Epub].


30. Estepp JH, Kalpatthi R, Woods G, Trompeter S, Liem RI, Sims K, et al. Safety and efficacy of voxelotor in pediatric patients with sickle cell disease aged 4 to 11 years. Pediatr Blood Cancer 2022;69:e29716.



31. Muschick K, Fuqua T, Stoker-Postier C, Anderson AR. Real-world data on voxelotor to treat patients with sickle cell disease. Eur J Haematol 2022;109:154–61.



32. Shah N, Lipato T, Alvarez O, Delea T, Lonshteyn A, Weycker D, et al. Real-world effectiveness of voxelotor for treating sickle cell disease in the US: a large claims data analysis. Expert Rev Hematol 2022;15:167–73.


33. Brown C, Idowu M, Drachtman R, Beaubrun A, Agodoa I, Nguyen A, et al. Patient-reported experiences in voxelotor-treated children and adults with sickle cell disease: a semistructured interview study. Biomed Res Int 2023;2023:7533111.




34. Glaros AK, Razvi R, Shah N, Zaidi AU. Voxelotor: alteration of sickle cell disease pathophysiology by a first-in-class polymerization inhibitor. Ther Adv Hematol 2021;12:20406207211001136.




35. Hebbel RP, Hedlund BE. Sickle hemoglobin oxygen affinity-shifting strategies have unequal cerebrovascular risks. Am J Hematol 2018;93:321–5.



36. Adams RJ, Brambilla D, Optimizing Primary Stroke Prevention in Sickle Cell Anemia (STOP 2) Trial Investigators. Discontinuing prophylactic transfusions used to prevent stroke in sickle cell disease. N Engl J Med 2005;353:2769–78.


37. Wang WC, Kovnar EH, Tonkin IL, Mulhern RK, Langston JW, Day SW, et al. High risk of recurrent stroke after discontinuance of five to twelve years of transfusion therapy in patients with sickle cell disease. J Pediatr 1991;118:377–82.


38. Voeks JH, Gray S, Lehrer-Graiwer J, Adams RJ. Correlation between hemoglobin levels and transcranial doppler velocities: a retrospective STOP 2 analysis in children with sickle cell disease. HemaSphere 2020;4(S1): 710–11.
39. Phan V, Hershenson J, Caldarera L, Larkin SK, Wheeler K, Cortez AL, et al. Effect of voxelotor on cardiopulmonary testing in youths with sickle cell anemia in a pilot study. Pediatr Blood Cancer 2023;70:e30423.


40. The effect of voxelotor on cerebral hemodynamic response in children with sickle cell Anemia (VoxSCAN) study [Internet]. Bethesda (MD): ClinicalTrials.gov; 2024 [cited 2024 Jun 10]. Available from: https://clinicaltrials.gov/study/NCT05018728.
41. An open-label extension study of voxelotor administered orally to participants with sickle cell disease who have participated in voxelotor clinical trials [Internet]. Bethesda (MD): ClinicalTrials.gov; 2024 [cited 2024 Jun 10]. Available from: https://clinicaltrials.gov/study/NCT04188509.
42. Study to assess the effect of long-term treatment with voxelotor in participants who have completed treatment in study GBT440-031 (034OLE) [Internet]. Bethesda (MD): ClinicalTrials.gov; 2024 [cited 2024 Jun 10]. Available from: https://clinicaltrials.gov/study/NCT03573882.
43. A phase 3, randomized, double-blind, placebo-controlled study of voxelotor (GBT440) in pediatric participants with sickle cell disease (HOPE Kids 2) [Internet]. Bethesda (MD): ClinicalTrials.gov; 2024 [cited 2024 Jun 10]. Available from: https://clinicaltrials.gov/study/NCT04218084.
44. Expanded Access Protocol for Adults and Pediatric Patients With Sickle Cell Disease Who Have No Alternative Treatment Options [Internet]. Bethesda (MD): Clinical Trials.gov; 2024 [cited 2024 Jun 10]. Available from: https://clinicaltrials.gov/study/NCT04724421.





 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link PubMed
PubMed Download Citation
Download Citation


