Article Contents
| Clin Exp Pediatr > Volume 68(9); 2025 |
|
Abstract
Background
Syncope is a temporary loss of consciousness due to cerebral hypoperfusion associated with autonomic dysfunction. Vasovagal syncope (VVS) and postural orthostatic tachycardia syndrome (POTS) are the most common causes of syncope in adolescents.
Purpose
Here we conducted a comparative analysis of VVS and POTS in adolescents using transcranial doppler (TCD) and autonomic function tests to identify the mechanisms underlying the occurrence of each.
Methods
From August 2014 to July 2024, a tilt-table test was conducted on patients who presented with syncope or presyncope as the main symptom. Based on the head-up tilt test results, the patients were classified into the VVS or POTS groups and their medical records retrospectively analyzed.
Results
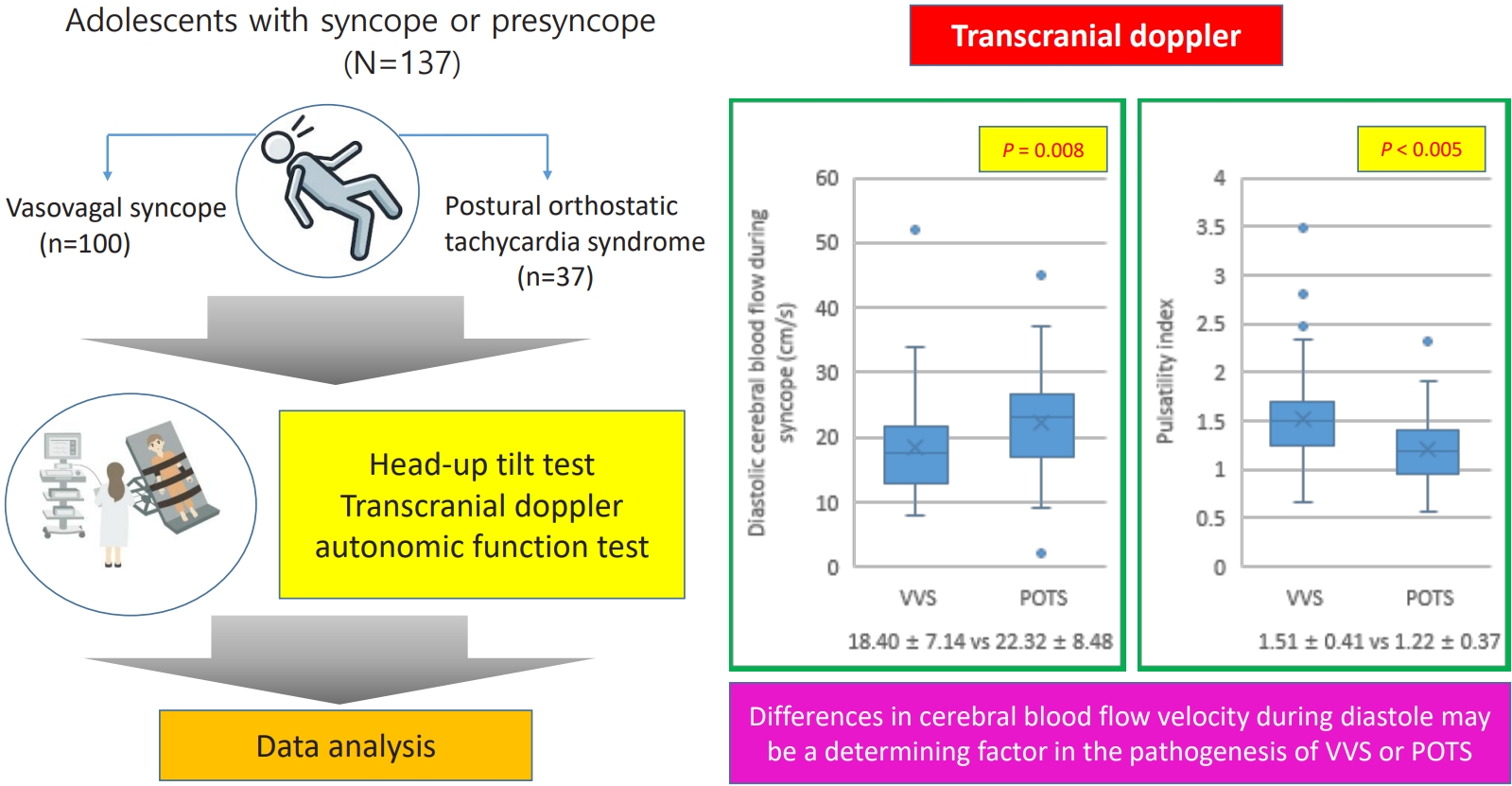
The study included 137 patients: 100 (73%) in the VVS group and 37 (27%) in the POTS group. There were no significant intergroup differences in patient characteristics. In the TCD, the diastolic blood flow velocity during symptom onset was significantly lower in the VVS versus POTS group (18.40±7.14 cm/sec vs. 22.32±8.48 cm/sec, P=0.008). Additionally, the pulsatility index was higher in the VVS group (1.51±0.41 vs 1.22±0.37, P<0.005). There were no intergroup differences in autonomic function tests results or composite autonomic severity scores.
Graphical abstract. VVS, vasovagal syncope; POTS, postural orthostatic tachycardia syndrome.
Syncope is a temporary loss of consciousness due to cerebral hypoperfusion and the inability to maintain posture and collapse due to loss of muscle tone, which is associated with autonomic dysfunction [1,2]. The characteristic symptoms of syncope include prodromal symptoms such as nausea, abdominal pain, dizziness, blurred vision, sweating, and palpitation when a person suddenly stands up or stands for a long time. Subsequently, the person loses consciousness and collapses but regains consciousness within a few minutes without any sequelae [1,3,4]. Approximately 15%–20% of adolescents experience one or more syncopal episodes, and approximately 3% of them visit the pediatric emergency room because of syncope. The overall incidence is approximately 0.5–3 per 1,000 people and is more common in girls, with the peak age being 15–19 years [4,5]. Syncope is categorized into neurally mediated, orthostatic, and cardiogenic syncope, with neurally mediated syncope being the most common [3,6]. The most common types of neutrally mediated syncope are vasovagal syncope (VVS) and postural orthostatic tachycardia syndrome (POTS).
VVS is characterized by hypotension and bradycardia when syncope occurs. Depending on the pattern and severity of hypotension and bradycardia, it is classified into mixed type, vasodepressor type, and cardioinhibitory type, with mixed type being the most common [1,4].
POTS is defined as dysautonomia affecting multiple somatic systems. It is diagnosed when a person has orthostatic intolerance for >3 months, has a heart rate (HR) of ≥120, and an HR increase of ≥30 compared with baseline in adults, or an HR increase of ≥40 in adolescents aged 12–19 years, without a decrease in blood pressure (BP) within 10 minutes after changing from a supine to an upright position [7-9].
Neurally mediated syncope can be diagnosed by history taking, physical examination, family history, and electrocardiogram, but history is limited because patients often do not remember the time of syncope [1,4]. Therefore, the head-up tilt test (HUT) can be performed for an accurate diagnosis and HUT is available for patients over 6 years of age [10]. Transcranial doppler (TCD) can be combined with HUT to improve diagnostic accuracy, and is useful in patients with orthostatic intolerance or hypotension [11]. Autonomic function tests can also be performed to assess the degree of autonomic dysfunction with the composite autonomic scoring scale (CASS) [12].
The mechanism of neurally mediated syncope has not been fully elucidated, but it involves a complex combination of the Bezold-Jarish reflex, sympathetic-parasympathetic imbalance, cerebral blood flow dysfunction, and biochemical substances such as noradrenaline, angiotensin II, arginine-vasopressin, serotonin, endogenous adenosine, and nitric oxide [3,13]. VVS and POTS are classified as neurally mediated syncope, yet there are distinct differences in the changes in BP and HR at the time of syncope. Although various hypotheses have been proposed regarding the pathogeneses of VVS and POTS, few studies have examined the differences between these 2 diseases. Therefore, this study aimed to compare and analyze the clinical features of VVS and POTS in adolescents and to compare TCD and autonomic function test results to determine the differences in the mechanisms of VVS and POTS.
From August 2014 to October 2024, this study was conducted on patients aged 8–18 years who visited Daegu Catholic University Hospital and Daegu Fatima Hospital for syncope. Their medical records were analyzed retrospectively and categorized into VVS and POTS groups, including only patients who tested positive on the HUT and experienced syncope or presyncope. Of a total of 186 patients, 49 were excluded from the study: 27 had incomplete data, 16 had negative HUT results, 3 had atrioventricular block, 2 had epilepsy, and 1 had a brain tumor.
Data for age, sex, height, weight, body mass index (BMI), prodromal symptoms and trigger factors, number of episodes, duration, and presence of headaches were collected and analyzed. Patients underwent HUT, TCD, and autonomic function tests early in the morning on the day after admission. Additionally, at the time of admission, BP, HR, complete blood count, liver function, electrolytes, thyroid function, iron levels, and N-terminal pro-B-type natriuretic peptide (NT-proBNP) were assessed. Chest x-ray, electrocardiogram, transthoracic echocardiography, electroencephalogram, and brain imaging (computed tomography or magnetic resonance imaging) were performed. All patients were followed up in the outpatient clinic to assess patients’ treatment response and prognosis.
The patient was bed-rested for at least 8 hours before being transferred to the HUT machine (TT-800s; Pampas, Korea). After lying in the supine position for 10 minutes, the patient was tilted 70°, and then BP and HR changes were measured every 5 minutes and monitored continuously for 30 minutes. If there was a significant decrease in BP and HR with presyncope and syncope symptoms during the observation period, the test was considered positive for HUT and stopped. However, if there was only dizziness, nausea, or headache, the test was continued. If the result was negative, the patient was placed in the supine position, medications such as isoproterenol or nitroglycerine were administered, and the test was continued according to the aforementioned protocol [12,14,15].
Autonomic neurologic function was assessed using the CASS, which scores based on 3 items: sudomotor index, adrenergic index, and cardiovascular HR index [12]. Sudomotor function is assessed using the quantitative sudomotor axon reflex test (Q-sweat, WR Medical Electronics Co., USA), which measures the humidity change due to sweat secretion after injecting acetylcholine solution into the skin at 4 sites: forearm, proximal leg, distal leg, and foot. The adrenergic index is analyzed by dividing the change in BP during the Valsalva maneuver into each period, and the cardiovascular HR index is evaluated with 2 components: Valsalva ratio and HR response to deep breathing [16-19].
The TCD examination and HUT were performed simultaneously. The cerebral blood flow velocities were measured at baseline (before tilting) and during syncope. After positioning the TCD probe (Pioneer System TC 2020; Nicolet Biomedical Inc., USA) in the transtemporal window, the systolic, diastolic, and mean cerebral artery blood flow velocities in the middle cerebral artery are continuously measured. The pulsatility index (PI) ([PI=V systole–V diastole]/V mean) was calculated using the measured values [11,13].
The chi-square test was used for comparisons of categorical data, mean and standard deviation values between the VVS and POTS groups were compared using an independent samples t test. All statistical analyses were performed using IBM SPSS Statistics ver. 25.0 (IBM Co., 4USA). Statistical significance was defined as P<0.05.
One hundred thirty-seven patients were included, of whom 100 had VVS and 37 had POTS. Patient age ranged from 9 to 18 years, the mean and standard deviation of age is 13.7±1.8 years. There were 46 males and 91 females. The 100 patients in the VVS group had a mean age and standard deviation of 13.7±1.8 years, with 35 males and 65 females. The POTS group had 37 participants, with a mean age and standard deviation of 13.7±1.6 years, 11 males and 26 females. There were no statistically significant differences in height, weight, BMI, systolic and diastolic BP, or HR on admission between the groups (Table 1). There were also no differences in complete blood count, liver function test results, electrolyte levels, thyroid function test results, iron levels, or NT-proBNP levels between the groups. All of the patients in this study had unremarkable findings on chest x-ray, electrocardiogram, transthoracic echocardiography, electroencephalogram, and brain imaging (computed tomography or magnetic resonance imaging).
Overall, syncope was most often triggered by a change in position. A postural change is when someone who was lying down suddenly stands up or someone who was sitting down suddenly stands up. The next cause was syncope from prolonged standing. Few patients experienced syncope while defecating, exercising, or experiencing stress (Fig. 1). There were no statistical differences in the triggering factors between the groups. In the study, patients had one or more prodromal symptoms. Prodromal symptoms of VVS included: dizziness (n=66), vision- related problem (n=47), headache (n=17), nausea/abdominal pain (n=16), weakness on extremity (n=11), chest discomfort/palpitation (n=8), tinnitus (n=5), cold sweating (n=2), and numbness in the legs (n=1). Prodromal symptoms of POTS included: dizziness (n=23), vision-related problem (n=24), headache (n=13), nausea/abdominal pain (n=6), tinnitus (n=5), chest discomfort/palpitation (n=3), weakness on extremity (n=2).
Before tilting the patient, there were no differences in diastolic blood flow velocity, mean blood flow velocity, and PI between the groups. Although there was no difference in mean blood flow velocity during syncope, diastolic blood flow velocity was statistically significantly lower in the VVS group (18.40±7.14 cm/sec) than in the POTS group (22.32±8.48 cm/sec) (P=0.008). The PI was higher in the VVS group than in the POTS group (1.51±0.41 vs. 1.22±0.37, P<0.005).
Regarding the change in TCD values before tilting the patient and at the onset of syncope in the HUT test, the change in diastolic blood flow velocity was significantly greater in the VVS group than in the POTS group (34.02±12.08 vs. 27.73±12.31, P=0.008), but there was no difference in the change in mean blood flow velocity and PI (Table 2).
There was no statistical difference in the sudomotor index, adrenergic index, cardiovascular index, Valsalva ratio, or HR response to deep breathing between the groups, and there was no difference in composite autonomic severity score between the groups (Table 3).
Of the total patients, 36 with VVS and 6 with POTS received lifestyle modification alone, while 64 with VVS and 31 with POTS received lifestyle modification along with medication. Lifestyle modification was implemented in all patients, but additional drug therapy was significantly more prevalent in POTS patients (P=0.025) (Table 4). Propranolol (Indenol, Dongkwang Pharmaceutical Co., Ltd., Korea) was used as a first-line agent. Among the 14 patients who did not respond to propranolol treatment, 12 were treated with midodrine (Midron, Myungmoon Pharm Co., Ltd., Korea) and 2 with fludrocortisone (Florinef, Samil Pharmaceutical Co., Ltd., Korea). Eight patients were treated with a combination of propranolol (Indenol, Dongkwang Pharmaceutical Co., Ltd.) and midodrine (Midron).
The median follow-up period for all patients was 7 months (range, 2–39 months). During the follow-up period, 59 patients in the VVS group experienced resolution of syncope and dizziness symptoms. However, 25 patients still had dizziness, and 10 patients had syncope. Among the 10 patients with syncope, 9 experienced only 1 episode of syncope. One patient had multiple episodes of syncope despite various medications and lifestyle modifications, and was ultimately diagnosed with panic disorder by a psychiatrist, with symptoms resolving after psychiatric treatment. In the POTS group, 22 patients experienced resolution of symptoms during follow-up. Ten patients continued to report dizziness after treatment, and among the 4 patients who experienced syncope, 2 patients had a single episode, while the remaining 2 patients had 2 episodes (Table 4).
Neurally mediated syncope can be considered autonomic dysfunction, and baroreceptor reflex and cerebrovascular autoregulation are important mechanisms that prevent neurally mediated syncope [3,13,20]. In this process, cerebrovascular autoregulation plays a crucial role. When syncope occurs, a decrease in cerebral blood flow precedes clinical changes in BP and HR. Cerebrovascular autoregulation normally functions such that when BP is low, the resistance of cerebral vessels decreases, causing the vessels to dilate, and when BP is high, the vessels constrict [4,21]. However, in patients with syncope, when blood flow decreases, the vessels actually constrict, leading to further reduction in blood flow. This phenomenon is referred to as paradoxical vasoconstriction [3]. Several studies have reported that cerebral artery constriction occurs during neurally mediated syncope, with no change in systolic cerebral blood flow velocity, but with a marked decrease in diastolic blood flow velocity and an increase in PI. PI is an indicator of vascular resistance, which increases when cerebral artery constriction occurs [13,22,23].
In the present study, we were able to confirm the decrease in cerebral blood flow by obtaining the change in blood flow and PI through TCD. In both VVS and POTS patients, cerebral blood flow volume decreased sharply before syncope occurred, followed by syncope within a few seconds.
VVS and POTS are representative of neurally mediated syncope, and their main mechanisms are similar. However, POTS is a cardiovascular autonomic disorder that differs from VVS. It is caused by a complex interaction of cardiac receptor-activating autoantibodies, hyperadrenergic activation, hypovolemia, and partial autonomic neuropathy [8,24-26], with impairment of cerebrovascular autoregulation being an important pathogenic mechanism. To differentiate between VVS and POTS, HUT is performed in conjunction with the TCD examination. This combination has higher diagnostic accuracy than HUT alone, which is helpful in diagnosing syncope [11]. In this study, the test results did not differ between the 2 groups except for those of the TCD examination. Additionally, there was no difference between the groups in the autonomic function test results. The TCD examination was performed during the HUT, and diastolic blood flow decreased more in the VVS group than in the POTS group; PI, which indicates the resistance of the blood vessels, was also higher in the VVS group than in the POTS group.
Based on these findings, the difference between VVS and POTS is not affected by different autonomic functions but rather by the degree of disturbance in cerebrovascular autoregulation. This indicates that impairment of cerebrovascular autoregulation was more severe in the VVS group than in the POTS group, and it can be assumed that VVS and POTS occur due to this difference in severity.
There are few comparative studies on cerebral blood flow between VVS and POTS in cases of impaired cerebrovascular autoregulation. In addition, the mechanisms by which cerebrovascular dysregulation in VVS and POTS cause reductions in cerebral blood flow have not yet been elucidated. When examining TCD changes in VVS and POTS, the blood flow velocity in the middle cerebral artery decreased by approximately 30% in VVS, and a prominent dicrotic notch pattern was observed. However, in POTS, the blood flow velocity decreased by 30%, and the duration of the decrease was longer than that of VVS. This pattern is associated with an increase in the PI, which is likely due to distal cerebral vasoconstriction caused by increased sympathetic nervous activity [11,27,28].
Although this study was able to determine the degree of difference in diastolic cerebral blood flow velocity between VVS and POTS, the duration of the decrease in cerebral blood flow velocity was not studied. If we can identify changes in the pattern of cerebral blood flow velocity decrease and recovery, we will be able to better understand the differences in cerebrovascular autoregulation impairment between VVS and POTS. A precise understanding of the mechanisms underlying VVS and POTS is essential for developing individualized treatment strategies [27]. Currently, VVS and POTS are managed with general treatment protocols, and treatment approaches are similar, with education and reassurance being key components.
Syncope prognosis is good and often improves within 1–2 years of syncope onset. Accurate diagnosis, reassurance, and lifestyle modifications can prevent syncope recurrence in approximately 50% of patients without medication and reduce the frequency of syncope by >70%. Even when syncope is treated with medication, 50%–70% of syncope resolves within 1–2 years [1,4]. The author of this study first educated patients about lifestyle modifications. The patients were instructed to drink 2 L of water per day, increase their sodium intake, and limit their caffeine intake. They were also instructed to wear tight-fitting pants and perform aerobic exercises and resistance training [1,4,29]. However, if the patients experienced more than 3 episodes of syncope, and the patients and their caregivers were very anxious about the syncope, medication was used. Although there are not many research results on medication-based treatment for children and adolescents, propranolol (Indenol), midodrine (Midron), and fludrocortisone (Florinef) can be used first [4,30].
The author used propranolol, a β-adrenergic blocker that lowers ventricular mechanoreceptor activity, which inhibits the increase in sympathetic tone. Midodrine is an α-adrenergic agonist, which induces venous and arterial vasoconstriction, resulting in increased systolic BP and decreased venous pooling. Fludrocortisone is a mineralocorticoid agonist that increases the plasma volume and preload by promoting renal sodium absorption [31]. In terms of symptom changes over the follow-up period of the patients in this study, it is challenging to draw precise conclusions due to the short follow-up period and statistical differences in the treatment methods of VVS and POTS. However, even during the short treatment period, many patients experienced a resolution of dizziness and syncope, and the study showed that the frequency of dizziness and syncope gradually decreased.
This study is meaningful because it compared and analyzed the mechanisms of VVS and POTS; however, it has some limitations. First, it was a retrospective study involving patients from 2 hospitals, and it was difficult to compare various data due to the small number of patients and limited data. However, the tests were performed by the same researcher using the same protocol, which increased the reliability of the comparative analysis. Second, while investigating the differences in the mechanisms of VVS and POTS, we were unable to perform various hematological tests. Although basic blood tests were conducted, levels of autoantibodies, norepinephrine, renin, and aldosterone were not measured in patients with POTS in this study. Including these tests would have helped further clarify the differences between VVS and POTS.
In this study, the only notable difference was that the diastolic blood flow in TCD was lower in VVS than in POTS, suggesting that the difference in the PI plays an important role in the occurrence of these conditions. The difference in the PI is associated with impairment in cerebrovascular autoregulation. To clarify this more concretely, research on cerebral blood flow and functional areas of the brain using functional magnetic resonance imaging and brain perfusion single photon emission computed tomography will be needed.
Footnotes
Fig. 1.
Trigger factors by study group. VVS, vasovagal syncope; POTS, postural orthostatic tachycardia syndrome.
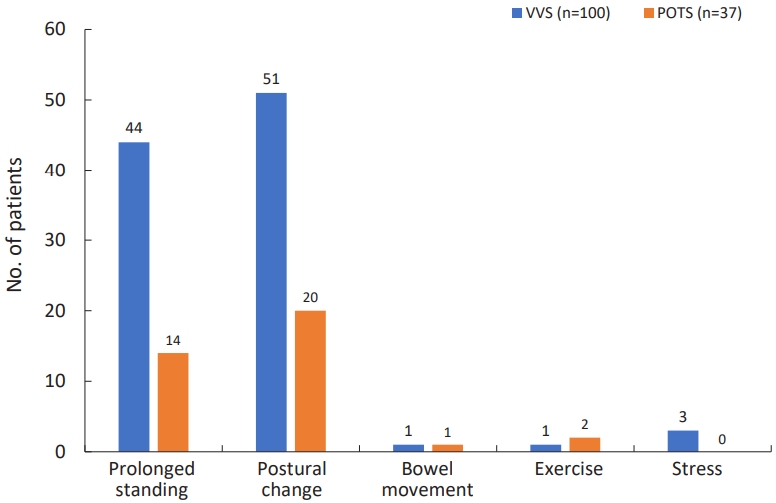
Table 1.
Patients' clinical characteristics by study group
Table 2.
Transcranial doppler results by study group
Table 3.
Autonomic function test results by study group
Table 4.
Treatment outcomes by modality and study group
References
1. Brignole M, Moya A, de Lange FJ, Deharo JC, Elliott PM, Fanciulli A, et al. 2018 ESC Guidelines for the diagnosis and management of syncope. Eur Heart J 2018;39:1883–948.

2. Van Dijk JG, Wieling W. Pathophysiological basis of syncope and neurological conditions that mimic syncope. Prog Cardiovasc Dis 2013;55:345–56.


4. Shen WK, Sheldon RS, Benditt DG, Cohen MI, Forman DE, Goldberger ZD, et al. 2017 ACC/AHA/HRS Guideline for the and management of patients with syncope. Circulation 2017;136:e60–122.

7. Boris JR, Moak JP. Pediatric postural orthostatic tachycardia syndrome: where we stand. Pediatrics 2022;149:e2021054945.


8. Bryarly B, Phillips LT, Qi F, Vernino S, Levine BD. Postural orthostatic tachycardia syndrome. J Am Coll Cardiol 2019;73:1207–28.


9. Boris JR. Postural orthostatic tachycardia syndrome in children and adolescents. Autono Neurosci 2018;215:97–101.

11. Cho BH. The pathophysiology of syncope and the role of transcranial doppler in its diagnostic evaluation. J Neurosonol Neuroimag 2024;16:51–62.


12. Chow KE, Dhyani R, Chelimsky TC. Basic tests of autonomic function. J Clin Neurophysiol 2021;38:252–61.


13. Sung RY, Du ZD, Yu CW, Yam MC, Fok TF. Cerebral blood flow during vasovagal syncope induced by active standing or head up tilt. Arch Dis Child 2000;82:154–8.



14. Cheshire WP, Goldstein DS. Autonomic uprising: the tilt table test in autonomic medicine. Clin Auton Res 2019;29:215–30.




17. Low PA, Tomalia VA, Park KJ. Autonomic function tests: some clinical applications. J Clin Neurol 2013;9:1–8.



18. Vogel ER, Corfits JL, Sandroni P, Sletten DM, Benarroch EE, Fealey RD. Effect of position on Valsalva maneuver: supine vs 20 degree position. J Clin Neurophysiol 2008;25:313–6.


19. Low PA. Composite autonomic scoring scale for laboratory quantification of generalized autonomic failure. Mayo Clin Proc 1993;68:748–52.


20. Longo S, Legramante JM, Rizza S, Federici M. Vasovagal syncope: an overview of pathophysiological mechanisms. Eur J Intern Med 2023;112:6–14.


21. Garcia A, Marquez MF, Fierro EF, Baez JJ, Rockbrand LP, Gomez-Flores J. Cardioinhibitory syncope: from pathophysiology to treatment-should we think on cardioneuroablation? J Interv Card Electrophysiol 2020;59:441–61.



22. Stewart JM. Postural tachycardia syndrome and reflex syncope: similarities and differences. J Pediatr 2009;154:481–5.



23. Claydon VE, Hainsworth R. Cerebral autoregulation during orthostatic stress in healthy controls and in patients with posturally related syncope. Clin Auton Res 2003;13:321–9.



24. Fedorowsk A. Postural orthostatic tachycardia syndrome: clinical presentation, aetiology and management. J Intern Med 2019;285:352–66.



25. Grubb AF, Grubb BP. Postural orthostatic tachycardia syndrome: new concepts in pathophysiology and management. Trends in Cardiovasc Med 2023;33:65–9.

26. Thieben MJ, Sandroni P, Sletten DM, Benrud-Larson LM, Fealey RD, Vernino S, et al. Postural orthostatic tachycardia syndrome: the Mayo Clinic experience. Mayo Clin Proc 2007;82:308–13.


27. Liao Y, Du J. Pathophysiology and individualized management of vasovagal syncope and postural tachycardia syndrome in children and adolescents: an update. Neurosci Bull 2020;36:667–81.









 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link PubMed
PubMed Download Citation
Download Citation


