< Previous Next >
Article Contents
| Korean J Pediatr > Volume 55(8); 2012 |
Abstract
Wheezing is one of the most frequent complaints that lead to the use of medical resources in younger children. Generally, wheezing is caused by bronchiolitis and resolves spontaneously without recurrence, but sometimes, wheezing can progress into asthma. Early data on the natural history of childhood wheezing was mostly obtained from retrospective reviews of medical records or from questionnaires, which made it difficult to exclude biases. Now that many cohort studies are available, reviewing the results of birth cohort studies makes it possible to understand the natural course of early childhood wheezing and the risk factors for asthma. In this study, we have reviewed the various phenotypes of early childhood wheezing and their natural courses to help select the most appropriate management modalities for the different types of early childhood wheezing.
Early data on the natural history of early childhood wheezing was mostly obtained by retrospectively reviewing medical records or by using questionnaires, and thus, these data were at inherent risk of being biased. Moreover, the issue of reverse causality, as well as a recall or selection bias, was hard to avoid. To overcome these risks, randomized prospective studies have been launched. Of these studies, birth cohort studies are very informative for gathering data on the natural history of early wheezing, the risk factors for asthma, and the effectiveness of the preventive modalities. In this study, we reviewed various findings from some large representative birth cohort studies (Fig. 1), which aided the understanding of the properties and natural courses of early childhood wheezing.
The most representative birth cohort study on early childhood wheezing is the Tucson Children's Respiratory Study (TCRS), which began in 1980 and included a large number of infants (n=1,246) enrolled at or soon after birth in Tucson, Arizona in the United States. It was designed as a longitudinal study to acquire information on the potential risk factors related to acute lower respiratory illness or chronic lung disease. The enrolled children were asked to answer respiratory questionnaires at the ages of 1, 3, 6, 11, and 16 years. Skin prick tests, blood tests for serum immunoglobulin E (IgE) and eosinophil counts, pulmonary function tests, and airway challenge tests were performed periodically. On the basis of the gathered information, the authors defined wheeze phenotypes in patients through the ages of 6 and 11 years and performed an extensive analysis of the wheezing data by incorporating variables related to genetics, environment, immunology, chemistry, and physiology1,2).
The usefulness of the information obtained from the TCRS led other centers to establish their own birth cohort studies. In 1989, researchers enrolled 1,456 infants born in the province of the Isle of Wight, United Kingdom (UK)3). They used questionnaires to obtain clinical information from participants at ages 1, 2, 4, 10, and 18 years; they also obtained the data for skin prick test (at ages 4, 10, and 18 years), serum IgE levels (at 10 years), bronchial challenge test (at 10 years), and lung function test (at 10 years). On the basis of their results, Kurukulaaratchy et al.3) summarized the characteristics and classification of the wheezing phenotypes. Recently, they also published an article on the effects of atopy and sex on rhinitis4).
The Multicenter Allergy Study (MAS) was launched in Germany in 1990 and enrolled 1,314 infants. Subjects were followed up periodically at 1, 3, 6, 12, 18, and 24 months of age, then annually until the age of 13 years. Lau et al.5) gathered information by using questionnaires, measured specific IgEs to both food and inhalant allergens (at ages 1, 2, 3, 5, 7, and 10 years), and performed lung function tests (at ages 7, 10, and 13 years). As a result, the MAS acquired extensive data, particularly with regard to the relationships between sensitization in early childhood and wheezing.
In 1995, the Manchester Asthma and Allergy Study (MAAS) was started in the UK. Infants (n=1,186) were enrolled and then evaluated using questionnaires at 1, 3, 5, and 8 years of age. Lung function tests were conducted at 3, 5, and 8 years, and skin prick tests and serum IgE measurements were performed at 1, 3, 5, and 8 years. Lowe et al.6) reported the relationship between preschool wheeze phenotypes and lung function results. Simpson et al.7) specified the atopy status in further detail (multiple-early, multiple-late, dust mite, non-dust mite) and reported a closer relationship between the multiple-early atopic phenotype and persistent wheezers. Their results were novel in that the types and numbers of allergens, as well as the age of sensitization, were differently related to wheezing phenotypes.
Two other studies are noteworthy. The Avon Longitudinal Study of Parents and Children (ALSPAC) enrolled 14,062 infants born in the province of Avon, UK, in 1991. Henderson et al.8) gathered clinical information annually via questionnaires from children aged 1 to 7 years and performed skin prick tests and lung function tests when subjects attained an age of 7 to 8 years. The Prevalence and Incidence of Asthma and Mite Allergy (PIAMA) study began in 1996 with 3,963 infants in the Netherlands. The subjects were aged between 1 and 8 years, and the authors interviewed caregivers annually, measured serum specific IgE antibodies (at ages 4 and 8), and assessed lung functions and airway hyper-responsiveness (AHR) at age 8 years9).
All of the abovementioned studies evaluated symptoms or events by using questionnaires, but tried to minimize recall bias by following up subjects in a short period. The investigated factors were similar across the studies but the time points and methods used to assess these factors were not identical, which may partly explain the discrepancy in relationships found between these studies. The number of subjects enrolled in the ALSPAC and the PIAMA study were greater than those in other studies; therefore, various wheezing phenotypes were observed, which enabled the authors to define wheezing phenotypes in further detail8,9).
The existence of various wheezing phenotypes and their distinctive characteristics provide an insight into the development of asthma and its natural history and imply that asthma is a complicated disorder with numerous underlying pathophysiological mechanisms10).
In the TCRS, based on clinical observations relating to the onset and persistence of early childhood wheezing, the subjects were classified into 4 wheezing phenotypes: transient-early, persistent, late-onset, and never wheezers11). When subjects experienced 1 or more wheezing episodes before the age of 3 years but not between the ages of 3 and 6 years, they were classified as transient-early wheezers. Those who had not exhibited wheezing by the age of 3 years but did so between the ages of 3 and 6 years were classified as late-onset wheezers. Persistent wheezers were defined as those who exhibited wheezing before 3 years of age and still had wheezing episodes between the ages of 3 and 6 years. The rest were classified as "never wheezers". Each phenotype showed different risk factors: transient-early wheezers had a history of maternal smoking, while in late-onset wheezers, maternal asthma was a risk factor, as was male sex and rhinitis in the first year of life. Maternal asthma, maternal smoking, rhinitis apart from colds, eczema in the first year of life, and male sex were independently predictive of persistent wheezing, and among these, maternal asthma was the most predictive. The fact that risk factors differed depending on phenotype implies that each phenotype reflects a pathophysiologically different disease entity. After assessing the results of the skin prick tests at age 6 years, the TCRS further divided wheezing phenotypes into 3 categories: transient-early, non-atopic, and IgE-associated wheezing/asthma, based on the results of a methacholine provocation test, diurnal variation of peak flow rate, and responses to a questionnaire relating to the presence or absence of wheezing/atopy12).
The Isle of Wight study, MAS, and MAAS adopted classifications systems similar to those of the TCRS3) but the age criteria were different: evaluations at the ages of 4 and 10 years13) were used for the Isle of Wight study, at 3 and 7 years for MAS14), and 3 and 5 years for MAAS. Familial history of asthma, recurrent respiratory infections at earlier ages, and allergic sensitization were the risk factors indicative of persistent wheezing. Wheezing in later childhood was also used as a criterion to classify subjects. In the Isle of Wight study, subjects who still had wheezing at 10 years were sub-classified as atopic and non-atopic wheezers and each had different risk factors; asthma in siblings, eczema in the first year of life, rhinitis at age 4, and male sex were more prevalent in atopic wheezers, whereas maternal asthma and recurrent respiratory infection at age 2 years were more prevalent in non-atopic wheezers13). In the MAS, wheezing at 13 years was used as a sub-classification, and was related to parental atopy, patient atopy, elevated total serum IgE at an early age, and excessive exposure to indoor allergens15). The MAAS measured specific airway resistance (sRaw) at early ages, which was included as a risk factor of wheezing persistence at the age of 5 years.
In the ALSPAC and the PIAMA study, the authors used the classification system used in the TCRS, but they also added distinctive subgroups. In the ALSPAC, prolonged-early wheezers and intermediate-onset wheezers were specified, whereas in the PIAMA study, only intermediate-onset wheezers were added. In these 2 studies, each phenotype had similar features not only with regards to prevalence but also in relation to atopic sensitization, pulmonary function, and AHR9).
According to epidemiologic studies, lung functions tend to be decreased in asthmatic adults and school-aged asthmatic children, and the decrease is associated with a history of childhood respiratory illnesses. Retrospective analysis cannot conclude whether childhood respiratory illnesses precede lung function decline or vice versa16). This lack of conclusion is related to the lack of tools available to assess pulmonary function readily and reliably in preschool children. A few studies have evaluated lung functions of younger children (maximal forced expiratory flow at functional residual capacity [V'max FRC] by rapid thoracic compression in the TCRS17) and sRaw by forced oscillation technique in the MAAS18)) but most studies have not evaluated lung functions until subjects reached the school-age. Considering that V'max FRC was reduced in recurrent wheezers who began wheezing within the first year of life, and that V'max FRC measured before 6 months was low only in transient-early but not in persistent wheezers, the decline in lung function seems to precede the onset of wheezing, and is not entirely attributable to the complications of respiratory illnesses. However, this needs to be confirmed by performing other cohort studies.
Lung functions measured repeatedly at different time points show a distinctive pattern according to each phenotype: lung function seems to be decreased in transient-early and persistent wheezers but not in late-onset or never wheezers. In the MAAS, sRaw was measured twice, at ages 3 and 5 years. sRaw measured at age 3 was significantly increased in persistent wheezers (but not in late-onset wheezers), and its elevation correlated with persistent wheezing at age 5 years6). V'max FRC measured at 6 years in the TCRS was lowest in persistent wheezers, followed by transient-early wheezers. This value was not decreased in late-onset wheezers compared to the never wheezers11) (Table 1). Lung function data measured using conventional spirometer is available from the age of 7 years. Except for the MAS, which reported decreased lung functions (forced expiratory volume in 1 second [FEV1], maximal expiratory flow at 50% capacity [MEF50%], MEF25%, and MEF75% at age 7 years14)) in persistent and late-onset wheezers but not in transient-early wheezers, studies have presented similar (but not identical) findings. FEV1 at age 8 years in the PIAMA study was lowest in persistent wheezers9), while FEV1 and forced expiratory flow between 25% and 75% of the forced vital capacity (FEF25-75) at age 8 to 9 years in the ALSPAC were decreased in prolonged-early, intermediate-onset, and persistent wheezers8). In the Isle of Wight study, FEV1 and forced vital capacity (FVC) measured at age 10 were reduced in both transient-early and persistent wheezers but were not different between groups when wheezers were divided into atopic and non-atopic subgroups. In the TCRS, lung functions (FEV1, FEF25-75, and FVC) measured at age 11 and 16 remained low in transient-early and persistent wheezers but not in late-onset wheezers12).
AHR has also been evaluated in these studies. AHR to eucapnic voluntary hyperventilation measured at age 5 in the MAAS was elevated in both persistent and late-onset wheezers but not in transient-early wheezers6). When AHR was assessed with methacholine, it was also more frequent in persistent and late-onset wheezers but not in transient-early wheezers3,12). Atopic wheezers (classified in the Isle of Wight Study) had higher prevalence of AHR3). In addition, intermediate-onset wheezers, a distinctive phenotype of the PIAMA and the ALSPAC studies also had a higher degree of AHR8,9). When AHR was assessed repeatedly, it gave further information on the relationship with clinical courses. In the TCRS, AHR was assessed twice: at ages 6 years (by using cold air) and 11 years (by using methacholine). AHR at age 6 years was related to the subsequent occurrence of asthma from the ages 6 to 11 years, but considering that the relationship was blunted after controlling for compounding factors19), AHR may be an indicator rather than an independent risk factor for the occurrence of asthma because of persistent allergic inflammation1). AHR at 11 years was more prevalent in subjects who had persistently wheezed at both ages 6 and 11 years and was also positively and independently associated with atopy at both 6 and 11 years.
Little data on peak expiratory flow (PEF) variability was reported in the cohort studies. In the TCRS, PEF variability measured at age 11 years increased in subjects who had exhibited wheezing at age 6 years, with the exception of those subjects who no longer reported wheezing at age 11 years. PEF variability, however, was not related to atopy at either age 6 or 11 years12).
In summary, while transient-early wheezers (but not late-onset wheezers) show decreased lung functions at early ages, late-onset wheezers (but not transient-early wheezers) have increased AHR. To date, whether decreased lung function will persist in later life is uncertain. In persistent wheezers, however, lung functions measured after the age of 6 years tend to decrease, which is indicative of airway remodeling, a long-term and well-known potential complication in asthmatic patients.
It is widely reported that approximately 80% of asthmatic children present with symptoms in the first few years of life, whereas about 30% of early wheezers develop medically diagnosed asthma1). Hence, early-stage discrimination between those who are likely to develop asthma and those who are not would be very useful in order to avoid overdiagnosis and/or overtreatment, and more importantly, to provide proper management and medical resources to those most at risk.
However, confirming asthma diagnosis is not easy in practice. Bronchiolitis manifests itself as a wheezing episode that cannot be discerned from asthma exacerbation. Spirometric values are sometimes unreliable and the acquisition of markers for eosinophilic inflammation directly from the airway is limited, especially with regard to infants, because this entails invasive procedures. Unfortunately, attempts to identify alternative variables, including genetic and other biomarkers, which are accurately predictive of asthma, have proved largely unsuccessful20). In addition, many attempts to predict asthma development using clinically measurable parameters and clinical features21,22) have failed to yield reproducible results with high odds ratios. Fortunately, with help from advanced technology enabling the measurement of airway inflammation and lung functions non-invasively in younger children, a prospective study investigating the relationships between these parameters and asthma at the age of 6 years has finally been initiated23). This study measures the levels of nitric oxide and volatile organic compounds from exhaled breath; levels of cytokines, chemokines, and adhesion molecules from exhaled breath condensate; and airway resistance before and after a bronchodilator and obtains a history on respiratory symptoms via a questionnaire on every visit.
As data accumulates, researchers have attempted to find various clinical indices or criteria that may predict the development of asthma later in the school-age from their own birth cohorts. The most representative index is the Asthma Predictive Index (API), which was derived from the TCRS24). According to this set of criteria, the diagnosis of asthma is predicted by the following: recurrent wheezing episodes more than 4 times previously, 1 of the 2 major criteria (physician-diagnosed eczema or parental asthma), or 2 of 3 minor criteria (physician-diagnosed allergic rhinitis, wheezing without cold, or peripheral eosinophilia concentration not less than 4%). Positive API values were highly predictive of current asthma between the ages of 6 and 13 years (as high as 77%), and negative values predicted a lack of it (68%). While this index had high specificity (as high as 97%), its sensitivity was low (15 to 22%), which is consistent with the fact that many of those who became asthmatic in adulthood experienced minimal wheezing during childhood. If we adopted the "loose index" (at least 1 wheezing episode during the first 3 years, without changing other criteria), the sensitivity would increase (up to 57%), although a greater proportion of patients who would not, in fact, become asthmatic later in life (i.e., transient-early wheezers) would be misclassified as positive and warrant unnecessary treatment25). The API was modified in accordance with the results of the Prevention of Early Asthma in Kids study as follows: more than 1 positive inhalant allergen added as a major criterion and food allergen sensitization (milk, eggs, and peanuts) were substituted for physician-diagnosed allergic rhinitis26).
The Isle of Wight study and the PIAMA study also suggested their own asthma predictive indices27,28). These indices emphasize the importance of familial history, recurrence of wheezing episodes, atopy, and concurrent allergic diseases. The Isle of Wight Index scores 4 items (familial history of asthma, recurrent respiratory infection at the age of 2 years, atopy at the age of 4 years, and recurrent rhinitis symptoms during the first year), and the PIAMA Index scores 7 items (male sex, post-term delivery, low parental educational status, parental history of inhaler medications, wheezing frequency, wheezing/dyspnea apart from colds, and physician-diagnosed eczema). A comparison of the indices used in the Isle of Wight and PIAMA studies with those used in the TCRS revealed that the Isle of Wight study exhibited similar sensitivity and specificity, and similar positive and negative predictive values to the TCRS. The PIAMA study yielded similar but higher negative and lower positive predictive values25).
Since these indices are derived from data obtained from specific birth cohorts, validation involving additional birth cohorts will be required prior to clinical application. Thus far, no subsequent data has emerged that supports the indices described in the Isle of Wight or PIAMA studies. Only the API (derived from the TCRS) has a supporting publication to date; when it was applied to the Columbian population, after a 6-year follow-up, it showed a sensitivity of 43% and a specificity of 79%, which is indicative of the possibility of sharing criteria between studies29). Therefore, guidelines, including those from the Global Initiative for Asthma, recommend consideration of the API when making a decision regarding the use of controller medications in wheezers less than 5 years old30). A Korean cohort study on the childhood origins of asthma and allergic diseases has been ongoing since 200831); it will be noteworthy to see whether the hypothesis derived from these previous birth cohort studies is replicated in the Korean population. Moreover, it will be possible to obtain more information on the prenatal and perinatal risk factors for asthma development in later childhood.
Five representative birth cohort studies have presented various but common phenotypes of early childhood wheezing, which indicates that early childhood wheezing is a complicated disorder with numerous pathophysiological mechanisms. Each study is valuable in its own right: the TCRS was the first such study and acted as a prototype, the Isle of Wight study extended the relationship between atopy and allergic diseases, while the MAAS measured lung function in very young children and suggested a novel approach for assessing atopic status. The MAS accumulated extensive pulmonary function data but the profile was different from that of the other studies. The ALSPAC and the PIAMA study have larger populations and thus have suggested more detailed phenotypes and risk factors. Although many discrepancies are present between them, these studies have presented similar wheezing phenotypes in terms of type and composition. Some indices produced by the studies have been regarded as good tools for the differentiation of subjects who will eventually
develop asthma. Now that Korean birth cohort studies have been launched, we expect the emergence of a new asthma predictive index that will be suitable for our own birth cohort population.
References
1. Taussig LM, Wright AL, Holberg CJ, Halonen M, Morgan WJ, Martinez FD. Tucson Children's Respiratory Study: 1980 to present. J Allergy Clin Immunol 2003;111:661–675.


2. Morgan WJ, Stern DA, Sherrill DL, Guerra S, Holberg CJ, Guilbert TW, et al. Outcome of asthma and wheezing in the first 6 years of life: follow-up through adolescence. Am J Respir Crit Care Med 2005;172:1253–1258.



3. Kurukulaaratchy RJ, Fenn MH, Waterhouse LM, Matthews SM, Holgate ST, Arshad SH. Characterization of wheezing phenotypes in the first 10 years of life. Clin Exp Allergy 2003;33:573–578.


4. Kurukulaaratchy RJ, Karmaus W, Raza A, Matthews S, Roberts G, Arshad SH. The influence of gender and atopy on the natural history of rhinitis in the first 18 years of life. Clin Exp Allergy 2011;41:851–859.


5. Lau S, Nickel R, Niggemann B, Gruber C, Sommerfeld C, Illi S, et al. The development of childhood asthma: lessons from the German Multicentre Allergy Study (MAS). Paediatr Respir Rev 2002;3:265–272.


6. Lowe LA, Simpson A, Woodcock A, Morris J, Murray CS, Custovic A, et al. Wheeze phenotypes and lung function in preschool children. Am J Respir Crit Care Med 2005;171:231–237.


7. Simpson A, Tan VY, Winn J, Svensen M, Bishop CM, Heckerman DE, et al. Beyond atopy: multiple patterns of sensitization in relation to asthma in a birth cohort study. Am J Respir Crit Care Med 2010;181:1200–1206.


8. Henderson J, Granell R, Heron J, Sherriff A, Simpson A, Woodcock A, et al. Associations of wheezing phenotypes in the first 6 years of life with atopy, lung function and airway responsiveness in mid-childhood. Thorax 2008;63:974–980.



9. Savenije OE, Granell R, Caudri D, Koppelman GH, Smit HA, Wijga A, et al. Comparison of childhood wheezing phenotypes in 2 birth cohorts: ALSPAC and PIAMA. J Allergy Clin Immunol 2011;127:1505–1512.e14.


11. Martinez FD, Wright AL, Taussig LM, Holberg CJ, Halonen M, Morgan WJ. Asthma and wheezing in the first six years of life. The Group Health Medical Associates. N Engl J Med 1995;332:133–138.


12. Stein RT, Holberg CJ, Morgan WJ, Wright AL, Lombardi E, Taussig L, et al. Peak flow variability, methacholine responsiveness and atopy as markers for detecting different wheezing phenotypes in childhood. Thorax 1997;52:946–952.



13. Kurukulaaratchy RJ, Fenn M, Matthews S, Arshad SH. Characterisation of atopic and non-atopic wheeze in 10 year old children. Thorax 2004;59:563–568.



14. Lau S, Illi S, Sommerfeld C, Niggemann B, Volkel K, Madloch C, et al. Transient early wheeze is not associated with impaired lung function in 7-yr-old children. Eur Respir J 2003;21:834–841.


15. Matricardi PM, Illi S, Gruber C, Keil T, Nickel R, Wahn U, et al. Wheezing in childhood: incidence, longitudinal patterns and factors predicting persistence. Eur Respir J 2008;32:585–592.


16. Burrows B, Knudson RJ, Lebowitz MD. The relationship of childhood respiratory illness to adult obstructive airway disease. Am Rev Respir Dis 1977;115:751–760.

17. Martinez FD, Morgan WJ, Wright AL, Holberg CJ, Taussig LM. Diminished lung function as a predisposing factor for wheezing respiratory illness in infants. N Engl J Med 1988;319:1112–1117.


18. Tepper RS, Morgan WJ, Cota K, Wright A, Taussig LM. Physiologic growth and development of the lung during the first year of life. Am Rev Respir Dis 1986;134:513–519.

19. Lombardi E, Morgan WJ, Wright AL, Stein RT, Holberg CJ, Martinez FD. Cold air challenge at age 6 and subsequent incidence of asthma. A longitudinal study. Am J Respir Crit Care Med 1997;156:1863–1869.


21. Palmer LJ, Rye PJ, Gibson NA, Burton PR, Landau LI, Lesouef PN. Airway responsiveness in early infancy predicts asthma, lung function, and respiratory symptoms by school age. Am J Respir Crit Care Med 2001;163:37–42.


22. Delacourt C, Benoist MR, Le Bourgeois M, Waernessyckle S, Rufin P, Brouard JJ, et al. Relationship between bronchial hyperresponsiveness and impaired lung function after infantile asthma. PLoS One 2007;2:e1180



23. van de Kant KD, Klaassen EM, Jobsis Q, Nijhuis AJ, van Schayck OC, Dompeling E. Early diagnosis of asthma in young children by using non-invasive biomarkers of airway inflammation and early lung function measurements: study protocol of a case-control study. BMC Public Health 2009;9:210



24. Castro-Rodriguez JA, Holberg CJ, Wright AL, Martinez FD. A clinical index to define risk of asthma in young children with recurrent wheezing. Am J Respir Crit Care Med 2000;162(4 Pt 1): 1403–1406.


25. Castro-Rodriguez JA. The Asthma Predictive Index: a very useful tool for predicting asthma in young children. J Allergy Clin Immunol 2010;126:212–216.


26. Guilbert TW, Morgan WJ, Zeiger RS, Bacharier LB, Boehmer SJ, Krawiec M, et al. Atopic characteristics of children with recurrent wheezing at high risk for the development of childhood asthma. J Allergy Clin Immunol 2004;114:1282–1287.


27. Kurukulaaratchy RJ, Matthews S, Holgate ST, Arshad SH. Predicting persistent disease among children who wheeze during early life. Eur Respir J 2003;22:767–771.


28. Caudri D, Wijga A, A Schipper CM, Hoekstra M, Postma DS, Koppelman GH, et al. Predicting the long-term prognosis of children with symptoms suggestive of asthma at preschool age. J Allergy Clin Immunol 2009;124:903–910.e1-7.


29. Castro-Rodriguez JA, Cifuentes L, Rodriguez-Martinez CE. The asthma predictive index remains a useful tool to predict asthma in young children with recurrent wheeze in clinical practice. J Allergy Clin Immunol 2011;127:1082–1083.


30. Pedersen SE, Hurd SS, Lemanske RF Jr, Becker A, Zar HJ, Sly PD, et al. Global strategy for the diagnosis and management of asthma in children 5 years and younger. Pediatr Pulmonol 2011;46:1–17.


31. Hong SJ. Childhood asthma and allergic diseases cohort study. Public Health Wkly Rep 2010;3:721–726.
Fig. 1
Major birth cohort studies mentioned in this paper and their time points of follow-up (vertical bars), based on the published articles. TCRS, Tucson Children's Respiratory Study; MAS, Multicenter Allergy Study; ALSPAC, Avon Longitudinal Study of Parents and Children; MAAS, Manchester Asthma and Allergy Study; PIAMA, Prevalence and Incidence of Asthma and Mite Allergy Study.
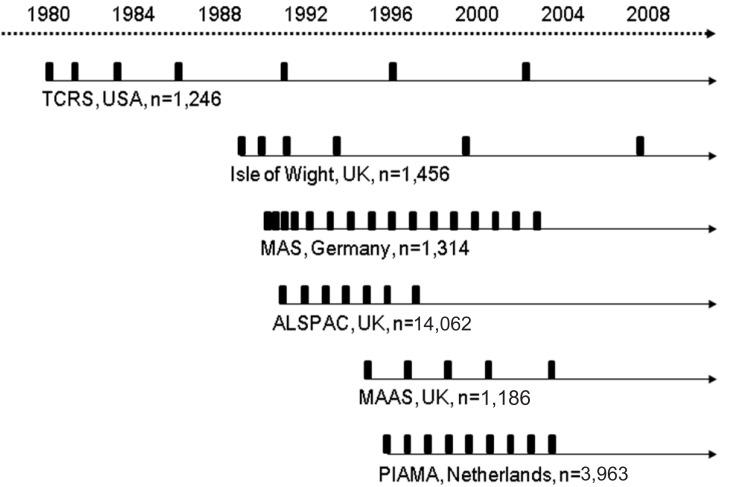
Table 1
Lung Function and Atopy Status according to Wheezing Phenotype in the Tucson Children's Respiratory Study
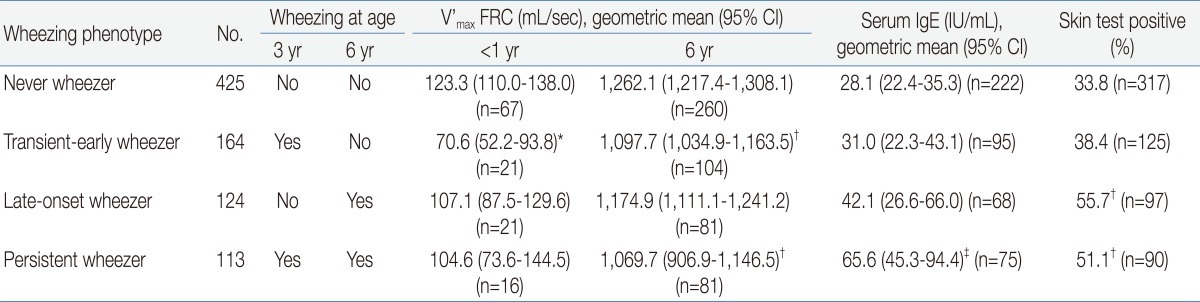
V'max FRC, maximal forced expiratory flow at functional residual capacity; CI, confidence interval; IgE, immunoglobulin E.
*P<0.01 for comparison with children who never wheezed and P<0.05 for comparison with children with late-onset wheezing and persistent wheezing. †P<0.01 for comparison with children who never wheezed. ‡P<0.01 for comparison with children who never wheezed and those with transient early wheezing.



 PDF Links
PDF Links PubReader
PubReader PubMed
PubMed Download Citation
Download Citation


