Introduction
Although leukemia is one of the most common neoplasms that metastasize to the heart, the occurrence of an intracardiac mass in acute leukemia is extremely rare1,2). The differential diagnosis of intracardiac mass lesions in leukemia should include intracardiac metastasis, thrombus, and vegetation2,3). An intracardiac mass may complicate a rare but fatal pulmonary embolism. Single pediatric case of primary cardiac lymphoma has been reported so far, but there are no reported pediatric cases of patients who presented with a cardiac mass resulting in a pulmonary embolism in South Korea4).
We hereby report the first case of a 13-year-old girl with acute lymphoblastic lymphoma-leukemia who presented with a cardiac mass in the right ventricle (RV) resulting in a pulmonary embolism.
Case report
A 13-year-old previously healthy girl presented to the emergency department with a right supraclavicular mass incidentally found one month prior to her arrival. The aspiration biopsy showed that it consisted of degenerative squamous cells with necrosis. At that time, she had a sore throat and felt feverish. The mass at the right supraclavicular area got bigger and tender three days prior to her arrival at Samsung Medical Center. An initial white blood cell (WBC) checked in the other hospital was 199,200×103/uL, and blasts were observed on the peripheral blood smear. An initial diagnostic work up for leukemia was started. She had no significant cardiopulmonary symptoms on admission.
There was no history of medical illness. Her birth history was unremarkable. Her family consists of her father, mother, older sister, and her twin brother. There was no family history of leukemia, congenital heart disease, or cancer.
The patient's height was 160.3 cm (50 to 75th percentile) and weight was 56.9 kg (50 to 75th percentile). On physical examination, her temperature was 37.9℃, heart rate was 115 beats/min, and respirations were 20/min. Blood pressure was 110/75 mmHg. She was noted to be ambulatory without assistance and in no apparent distress. She was awake, alert, and speaking in full sentences.
There was a palpable lymph node at the right supraclavicular area; it was 1×2 cm in size with tenderness upon palpation and movable. It had no redness and was not warm to the touch. There was another small palpable lymph node. Cardiac examination revealed regular tachycardia and a nondisplaced point of maximal impulse. There were no murmurs, rubs, gallops, or jugular venous distension. Chest wall movement was symmetrical, with clear and equal breath sounds. There were no motor or sensory deficits. She exhibited good coordination. The remainder of the physical examination was unremarkable.
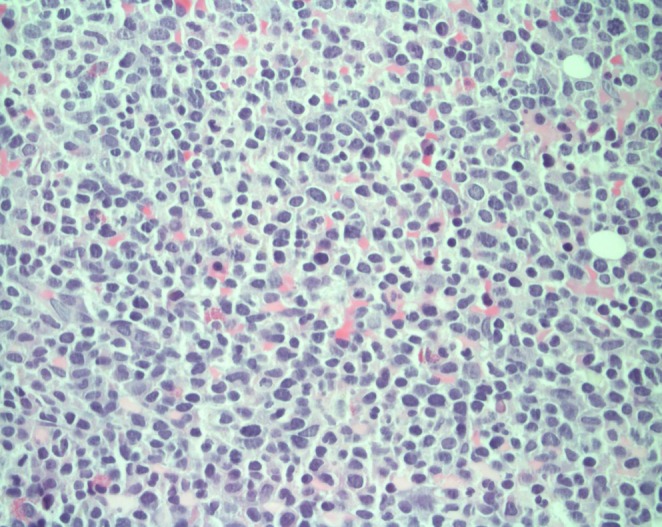
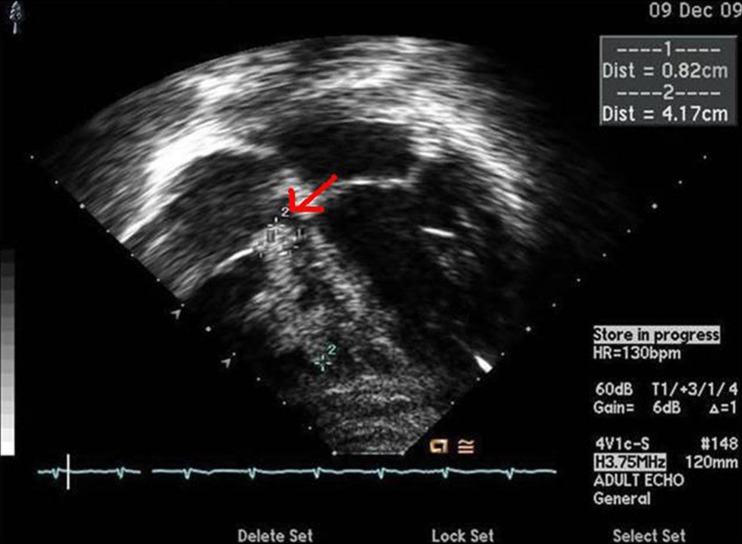
Bone marrow analysis showed blasts of 30%, which was compatible with acute leukemia (Figs. 1, 2). The chest radiograph was normal, and the electrocardiogram showed normal sinus rhythm. Two-dimensional echocardiography before chemotherapy revealed a 16×18×18 mm sized cardiac mass that was highly mobile and attached to the right ventricular free wall and chordae of the tricuspid valve. Its shape was bizarre, with heterogeneous echogenecity (Fig. 3). The mass looked like an avascular mass on cardiac magnetic resonance imaging (Fig. 4). RV thrombus, vegetation, or metastasis were considered as possible etiologies. To rule out cardiac vegetation and diagnose infective endocarditis, blood cultures were done, but no organism was isolated. A blood test to rule out thromboembolism was conducted: D-dimer was 60 ug/mL, and another coagulation test, International Normalized Ratio, was 1.52 (reference range, 0.9 to 1.1). In addition, fibrinogen was 87 mg/dL (reference range, 182 to 380) and antithrombin III activity was 67% (reference range, 83 to 123). There was no bleeding tendency.
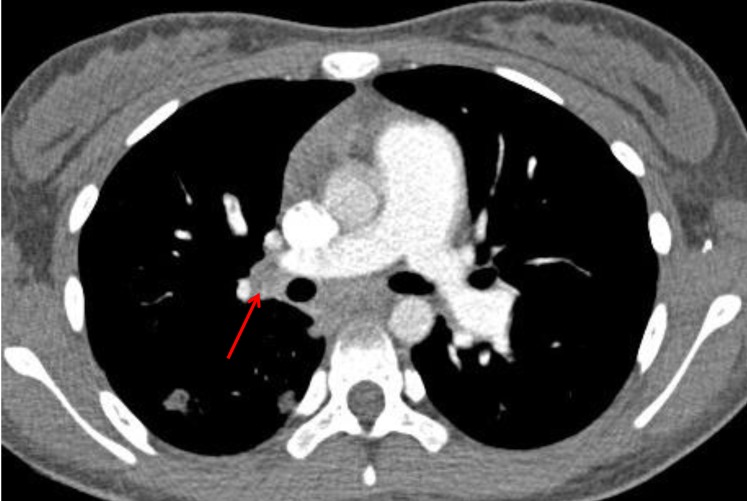
The patient received intravenous antibiotics and antifungal agents in order to treat possible infective endocarditis with vegetation. Heparinization was also started for possible thrombus. Treatment for acute leukemia including intravenous hydration and alkalization, plasmapheresis, and low dose oral prednisolone was started along with other treatments. On the following day, she developed sudden chest pain and dyspnea, which turned out to be caused by pulmonary embolism, which was discovered by chest computed tomography (Fig. 5). Despite heparinization and antibiotics, the RV mass size appeared to grow, as shown by a follow-up 2-dimentional echocardiogram. She was transferred to the pediatric intensive care unit because of progressive dyspnea. Sudden hemodynamic collapse developed the next morning. Extracorporeal membrane oxygenation (ECMO) support and emergency surgery was performed due to acute pulmonary embolism and for removal of the cardiac mass.
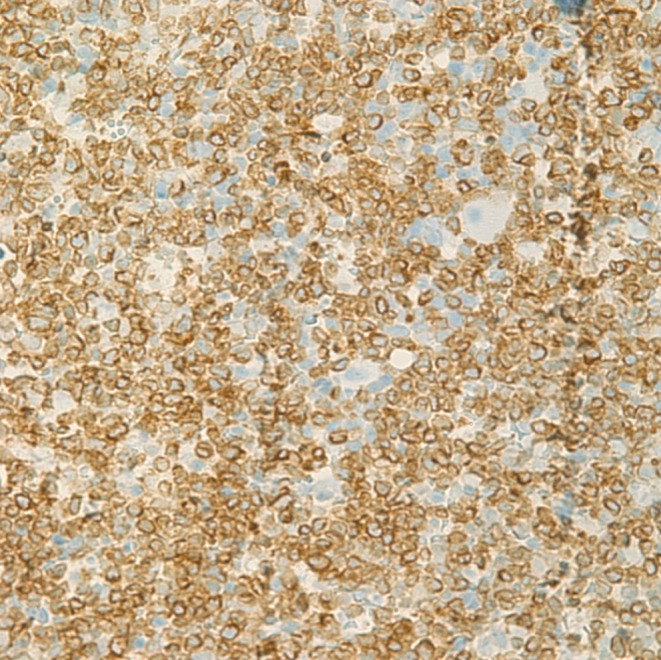
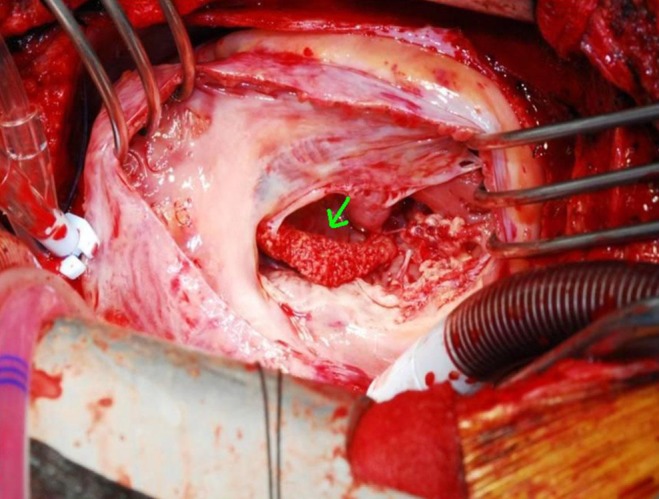
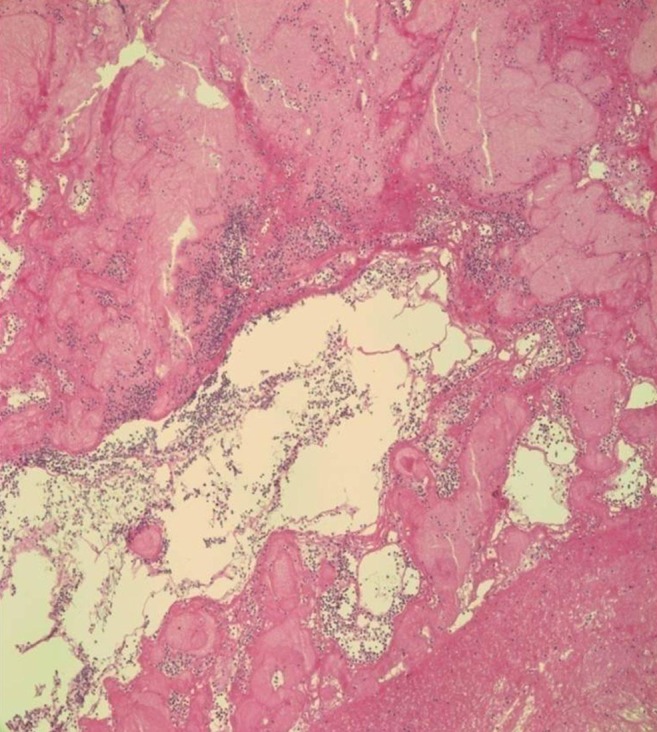
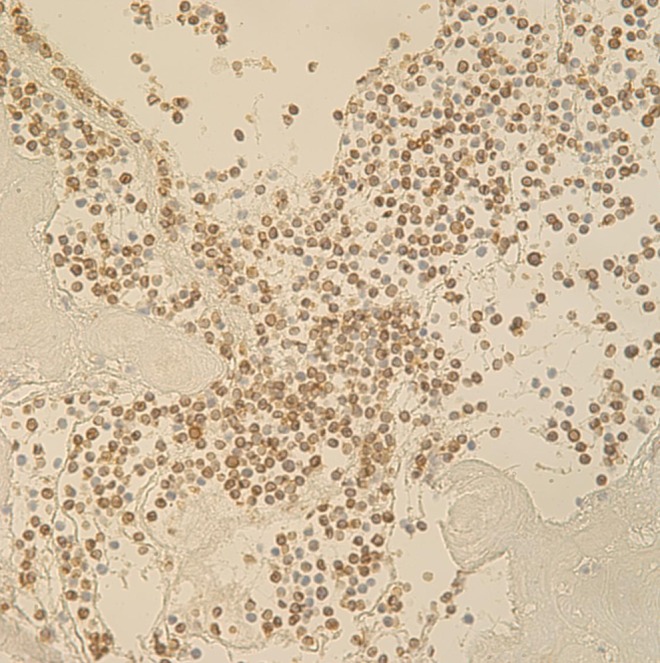
On surgical findings, the mass was fragile and easily crumbled and attached to a dysplastic tricuspid valve (Fig. 6). Also, irregular shaped masses were found in both pulmonary arteries. Pathology revealed that the cardiac mass and pulmonary artery mass consisted of an aggregation of small round necrotic cells that were compatible with leukemic cells (Figs. 7, 8).
In spite of aggressive postoperative ECMO support, the patient died of disseminated intravascular coagulation and heart failure the day after surgery.
Discussion
This is the first case of acute lymphoblastic leukemia presenting as a right ventricular mass and consequent fatal acute pulmonary embolism. This case had difficulties in the differential diagnosis and choosing appropriate management.
In acute leukemia, immature cells commonly infiltrate the myocardium, causing conduction abnormalities and rhythm disturbances5). There have been only a few cases of B-cell lineages as masses reported in the literature. Cardiac infiltration is very rare, and in leukemia only a few cases of pericardial effusion, heart muscle infiltration, or intracavitary masses have been described. Cardiac metastases from nonsolid tumors secondary to primary leukemia have accounted for 4.4%6). Although cardiac masses in recently reported cases of leukemia are in the right side of the heart, there has been no evidence of a common site for cardiac masses. A high WBC count in leukemia is reported to have a high risk for leukemic cell aggregation and a cardiac mass, and most patients who have had a cardiac mass diagnosis have had acute myeloid leukemia (AML)2,6). Cardiac masses in acute lymphoblastic leukemia are not common. A case of intracardiac thrombi in AML was reported in adults2). There are no reported cases that presented with cardiac mass resulting in pulmonary embolism in leukemic children in South Korea.
Depending upon the location, cardiac mass may obstruct the right or left ventricular outflow, resulting in dyspnea, chest pain, congestive heart failure, or syncope. Because leukemic tumors are reported to be fragile enough to cause a disseminated life threatening pulmonary embolism, cautious and earlier aggressive treatment should be considered in case of suspicious cardiac mass of leukemia.
The mechanism for increased risk of thromboembolism in acute leukemia is associated with alteration in the hemostatic system7). Approximately 90% of thromboembolism cases occur during induction, and the remaining 10% occur during consolidation or therapy intensification protocols. The recently published meta-analysis by Caruso et al.8) did not demonstrate a statistically significant difference in venous thromboembolism prevalence according to the corticosteroid type and dexamethasone used in induction. Therefore, in the patient with acute leukemia with venous thromboembolism, primary prophylaxis is warranted during chemotherapy agent exposure.
Laboratory findings and imaging studies could not give a definite diagnosis for this cardiac mass. There was no helpful imaging study to aid in the differential diagnosis. Lack of definite diagnostic evidence necessitated treatments covering all possible causes of cardiac mass and underlying disease of acute lymphoblastic leukemia. Rapid deterioration related to pulmonary embolism might have been related to the effect of the chemotherapy agent (prednisolone), which made the cardiac leukemic mass disseminate into pulmonary embolism.
In conclusion, cardiac mass in a child with acute leukemia should be investigated to rule out every possible causes, including vegetation, thrombus, and even cardiac mass of leukemic cells resulting in the fatal complication of pulmonary embolism. Clinicians should be alert to the possibility of the rapid progression to pulmonary embolism after chemotherapy.




 PDF Links
PDF Links PubReader
PubReader PubMed
PubMed Download Citation
Download Citation


