Introduction
Organotypic slice culture has several advantages, including preservation of the gross cytoarchitecture and the possibility of long-term observation and manipulation, and is frequently used to examine physiologic or pathologic processes1). The hippocampus is a major component of the brain. It belongs to the limbic system and plays important roles related to storing new memories. The hippocampus is the most susceptible brain region to various injuries. It has a simple anatomic structure and is easy to manipulate. As a result, organotypic hippocampal slice culture is commonly used to study neuronal cell death, neuroprotection, and synaptic plasticity2).
Newborn infants are prone to developing hypothermia3), which can result in metabolic acidosis, hypoxia, hypoglycemia, and multiorgan damage (e.g., pulmonary hemorrhage, renal failure, and disseminated intravascular coagulopathy) in neonates4,5). Hypothermia can be fatal, particularly in preterm infants. The mortality rate is approximately 10%, and approximately 10% of the survivors show evidence of brain damage3).
The immature brain is more vulnerable to unconjugated bilirubin, hypoxic-ischemic damage and acute hypoglycemia than the mature brain6-8). However, some studies have shown that the immature brain is more resistant to several types of injury, including N-methyl-D-aspartate (NMDA) toxicity and glutamate- and seizure-induced hippocampal damage, than the mature brain9-11). Although hypothermia is known to cause brain damage, few studies have investigated differences between the mature and immature brain as a result of hypothermic injury12,13). According to previous study, 7 days in vitro (DIV) hippocampal tissues are representative of the immature brain, and 14 DIV hippocampal tissues are representative of the mature brain1). Prominent damage to hippocampal tissues and loss of hippocampal neurons have been described in several experiments evaluating hypothermic injury13). The present study measured the damage to immature and mature hippocampal tissues following hypothermic exposure.
Materials and methods
1. Organotypic slice culture
Organotypic slice cultures of the hippocampus were prepared from a total of 12 7-day-old Sprague Dawley rats (DooYeol Biotech, Seoul, Korea) using the Stoppini method14). The rats were stabilized for 24 hours prior to the experiment and decapitated using scissors. The heads were sterilized with 70% alcohol, and the hippocampi were quickly removed and placed in ice-cold Gey's Balanced Salt Solution (GBSS, Sigma Aldrich, St Louis, MO, USA). The hippocampi were sliced (450 µm thick) with a tissue slicer (Stoelting Co., Wood Dale, IL, USA). Millicell culture inserts (Millipore, Ireland, 5 slices per insert) were placed into 6-well plates (SPL Life Sciences Co., Pocheon, Korea) with 1 mL of Gahwiler media15) per well. The slice culture media consisted of 25% Hank's Balanced Salt Solution (HBSS, GibcoBRL/Life Technologies, USA), 25% heat inactivated horse serum (Hyclone, Logan, UT, USA), 50% Basal Medium Eagle (BME, GibcoBRL/Life Technologies, Grand Island, NY, USA), 6.5 mg/mL glucose, and 200 mM glutamax-I (GibcoBRL/Life Technologies). The slices were cultured at 37℃ with 5% CO2 in a humidified incubator (MCO175, Sanyo, Tokyo, Japan). The media was changed twice per week, and all procedures were performed aseptically in a horizontal flow hood.
2. Hypothermic injury
The slices were divided into two groups, 7 DIV and 14 DIV. The tissues in each group were maintained for 0, 10, 30, and 60 minutes at 25℃ (0 minute, control; n=30 slices per subgroup) and rewarmed at 37℃ with 5% CO2 in a humidified incubator. Deteriorating tissues were excluded prior to induction of hypothermic injury.
3. Image analysis
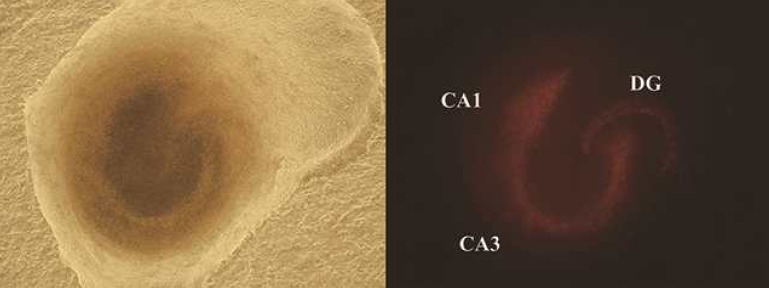
Fluorescent images of the slices were captured under an inverted microscope (IX 71, Olympus, Japan) 24 and 48 hours after hypothermic injury. At 72 hours after hypothermic injury, the slices were exposed to 2 mM NMDA in serum free medium for 30 minutes at 37℃ with 5% CO2 in a humidified incubator. The slices were cultured with 1 mL of serum free media with 1 µg propidium iodide (PI; Sigma Aldrich), and fluorescent images were obtained 96 hours after hypothermic injury. The images were analyzed with Image J (version 1.47c, National Institutes of Health, Bethesda, MD, USA). We defined threshold as amount of fluorescent area and a single researcher measured the individual areas of the cornu ammonis 1 (CA1), CA3, and dentate gyrus (DG) above the threshold (Fig. 1). After NMDA processing, the images were assumed to include the total area of the hippocampal tissue. We estimated the extent of damage 24 and 48 hours after hypothermic injury. The tissues in the 7 DIV and 14 DIV categories were compared, and the cold exposure groups (10, 30, and 60 minutes) in each category were compared with the control group. The ratio of damaged area at 24 and 48 hours to the total damaged area after NMDA treatment was calculated.
4. Statistical analysis
We evaluated brain injury by comparing tissues at 7 DIV with tissues at 14 DIV using the Student t test and Mann-Whitney U test. Repeated analysis of variance was used to assess the extent of injury based on the length of time after hypothermic injury and rewarming. Differences were considered significant when P<0.05. All analyses were performed using IBM SPSS ver. 18.0 (IBM Co., Armonk, NY, USA).
Results
1. Cultured hippocampal tissues at 7 DIV
1) Cold exposed groups compared with the control group
Damage to CA1 in the hippocampal tissue at 7 DIV was 0.00±0.00 and 0.01±0.02 in the control group at 24 and 48 hours, respectively (mean±standard deviation, n=30, measured area/total area). In the tissues exposed to cold for 10 minutes, the damage was 0.00±0.00 and 0.30±1.46 at 24 and 48 hours. In the tissues exposed for 30 minutes, the damage was 0.04±0.20 and 0.32±1.18 at 24 and 48 hours. In the tissues exposed for 60 minutes, the damage was 0.11±0.35 and 3.21±9.52 at 24 and 48 hours. There were no significant differences between the tissues exposed to cold for 10 minutes and the control group (P=0.169 and P=0.363) and 30 minutes and the control group (P=0.361 and P=0.307) at 24 and 48 hours. The tissues exposed for 60 minutes showed increased damage compared to the control group at 48 hours (P<0.001).
Damage to CA3 in the hippocampal tissue at 7 DIV was 0.00±0.01 and 0.00±0.01 in the control group at 24 and 48 hours, respectively. In the tissues exposed to cold for 10 minutes, the damage was 0.00±0.00 and 0.00±0.00 at 24 and 48 hours. In the tissues exposed for 30 minutes, the damage was 0.00±0.0 and 0.03±0.10 at 24 and 48 hours. In the tissues exposed for 60 minutes, the damage was 0.01±0.02 and 1.20±3.97 at 24 and 48 hours. There were no significant differences between the tissues exposed to cold for 10 minutes and the control group (P=0.185 and P=0.052) at 24 and 48 hours and 30 minutes and the control group (P=0.056 and P=0.852) at 24 and 48 hours. The tissues exposed for 60 minutes showed a significant increase in damage compared to the control group at 48 hours (P=0.005).
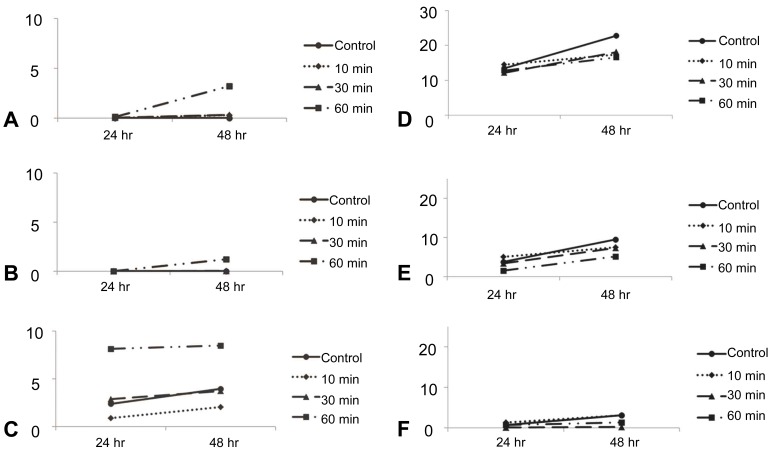
Damage to DG in the hippocampal tissue at 7 DIV was 2.38±8.46 and 3.95±9.22 in the control group at 24 and 48 hours, respectively. In the tissues exposed to cold for 10 minutes, the damage was 0.88±2.26 and 2.05±3.66 at 24 and 48 hours. In the tissues exposed for 30 minutes, the damage was 2.87±8.05 and 3.72±9.28 at 24 and 48 hours. In the tissues exposed for 60 minutes, the damage was 8.14±11.84 and 8.48±10.80 at 24 and 48 hours. The tissues exposed for 10 minutes (P=0.585 and P=0.538) and 30 minutes (P=0.077 and P=0.153) did not show significant differences compared with the control group at 24 and 48 hours. The tissues exposed for 60 minutes showed a significant increase in damage compared to the control group at 24 and 48 hours (P=0.008 and P=0.011). In summary, the tissues exposed for 60 minutes showed more damage to the CA1 and CA3 than the control group at 48 hours. Damage to DG was evident at 24 and 48 hours (Fig. 2).
2) Changes in damage over time
Damage to CA1 in the control group and tissues exposed for 10, 30, and 60 minutes in the hippocampal tissues at 7 DIV showed increased damage between the 24 and 48 hours time points after hypothermic injury (P=0.049). However, there was no significant increase in damage to CA3 and DG over time (CA3, P=0.107; DG, P=0.710) (Fig. 2)
3) Damage and duration of exposure
On CA1 of 7 DIV, there were significant difference between control group, 10, 30, and 60 minutes exposed groups (P=0.040). On CA3 of 7 DIV, there were no significant difference between control group, 10, 30, and 60 minutes exposed groups (P=0.066). On DG of 7 DIV, also significant difference was observed between control group, 10, 30, and 60 minutes exposed groups (P=0.013).
In the CA1 category, there were significant differences in damage between the control group, and the group of tissues exposed for 10, 30, and 60 min (P=0.040). In the CA3 category, there were no significant differences in damage between the control group, and the group of tissues exposed for 10, 30, and 60 minutes (P=0.066). Significant differences were observed between the control group, tissues exposed for 10, 30, and 60 minutes in the DG category (P=0.013) (Fig. 2).
2. Cultured hippocampal tissues at 14 DIV
1) Cold exposure groups compared with the control group
Damage to CA1 in the hippocampal tissue at 14 DIV was 13.40±17.34 and 22.75±22.46 in the control group at 24 and 48 hours, respectively. In the tissues exposed to cold for 10 minutes, the damage was 14.48±32.13 and 17.41±26.89 at 24 and 48 hours. In the tissues exposed for 30 minutes, the damage was 12.12±19.03 and 18.09±22.20 at 24 and 48 hours. In the tissues exposed for 60 minutes, the damage was 12.76±20.03 and 16.54±18.93 at 24 and 48 hours. There were no significant differences between the control group and the tissues exposed for 10 minutes (P=0.849 and P=0.367), 30 minutes (P=0.767 and P=0.387), and 60 minutes (P=0.892 and P=0.267) at 24 and 48 hours.
Damage to CA3 in the hippocampal tissue at 14 DIV was 3.75±9.69 and 9.48±15.95 in the control group at 24 and 48 hours, respectively. In the tissues exposed to cold for 10 minutes, the damage was 5.06±15.89 and 7.47±21.40 at 24 and 48 hours. In the tissues exposed for 30 minutes, the damage was 3.34±11.40 and 7.31±16.36 at 24 and 48 hours. In the tissues exposed for 60 minutes, the damage was 1.49±3.36 and 5.13±10.18 at 24 and 48 hours. There were no significant differences between the control group and the tissues exposed for 10 minutes (P=0.656 and P=0.648), 30 minutes (P=0.868 and P=0.575), and 60 minutes (P=0.302 and P=0.251) at 24 and 48 hours.
Damage to DG was 0.64±3.25 and 3.08±10.43 in the control group at 24 and 48 hours, respectively. At 24 and 48 hours, the damage was 1.31±4.16 and 3.05±7.50 in the tissues exposed to cold for 10 minutes, 0.05±0.10 and 0.18±0.37 in the tissues exposed for 30 minutes, and 0.69±2.09 and 1.30±2.88 in the tissues exposed for 60 minutes. There was no significant difference between the control group and the tissues exposed for 10 min (P=0.443 and P=0.988) and 60 minutes (P=0.954 and P=0.446). The tissues exposed for 30 minutes showed significantly less damage at 48 hours than the control group (P=0.361 and P=0.048) (Fig. 2).
2) Changes in damage over time
Regarding CA1, CA3, and DG, all of the tissues at 14 DIV showed significant deterioration between the 24 and 48 hours time points (CA1, P<0.001; CA3, P<0.001; DG; P=0.025). However, these results are not relevant because the tissues at 14 DIV were not significantly damaged compared with control group (Fig. 2).
3) Damage and duration of exposure
There were no significant differences in damage between the control group and the groups of tissues exposed to cold for 10, 30, and 60 minutes in all the categories of CA1, CA3, and DG at 14 DIV (CA1, P=0.182; CA3, P=0.558; DG, P=0.321). All of these results were time compensated. In summary, there were no statistically significant differences in the duration of hypothermic exposure in the 7 DIV or 14 DIV groups (Fig. 2).
Discussion
Cold injury in neonates and infants can cause death, multiple systemic dysfunction, and neurodevelopmental disturbances. Culic5) suggested that the infant brain is extremely vulnerable to deep hypothermia. The aim of this study was to determine the effects of hypothermic injury on the immature and mature brain. Neurons at 7 and 14 DIV represented the immature and mature brain, respectively. In hippocampal tissues at 7 DIV, the CA1 and CA3 showed an increase in damage after 60 minutes of exposure compared to the control group at 48 hours. DG showed increased damage at 24 and 48 hours. Hippocampal tissue at 14 DIV demonstrated no significant differences compared with the control group, except for the tissues exposed for 30 minutes in which decreased damage was shown compared to the control group at 48 hours. In general, the mature neurons showed less damage as a result of hypothermic injury than the immature neurons compared to the control groups, although direct comparisons are limited. According to Warren et al.13), hippocampal neuronal injury from hypothermia and rewarming is primarily related to intracellular Ca2+ accumulation mediated by NMDA receptors. Neuronal expression of different glutamate receptor subtypes is highly dependent on developmental stage, and responses to glutamate differ between immature and mature neurons9,10,16). These studies could explain the increased damage in immature neurons in response to hypothermic injury, as shown in this study. The effects of hypothermic injury on the immature brain compared to the mature brain are in contrast to the results of previous studies based on NMDA toxicity and glutamate- and seizure-induced hippocampal damage9-11). Additional studies should be performed to evaluate the possible involvement of different glutamate receptor subtypes or unknown mechanisms.
In a clinical setting, induced mild hypothermia (brain temperature of 32℃-34℃) is applied for therapeutic purposes to treat neonatal hypoxic ischemic brain damage17,18) or adult cardiac arrest19). Further studies are needed to determine the most effective, safe target temperatures and duration of exposure on long-term neurodevelopmental outcomes, particularly in neonates because the immature brain is vulnerable to hypothermia, as indicated in the present study. Body temperature should be carefully monitored and hypothermia should be avoided at birth or during invasive procedures.
Although mature hippocampal tissues experienced gradual damage over time compared to the control group, immature hippocampal tissues only showed statistical significance in damage to CA1.
In this study, the cold exposure times were 0, 10, 30, and 60 minutes. There were statistical differences in the extent of damage to CA1 and DG in tissues at 7 DIV exposed for 0, 10, 30, and 60 minutes. Significant differences between the duration of cold exposure were not observed in tissues at 14 DIV. Although 25℃ is considered profound hypothermia, any exposure to hypothermia can be harmful, and the duration of exposure affects the immature brain in particular.
In conclusion, hypothermia can damage systemic organs and the brain. Because the immature brain is more vulnerable to hypothermic injury than the mature brain, hypothermia should be carefully avoided, particularly in neonates. Studies on therapeutic hypothermia, effective temperatures, and duration of treatment should be performed.
Future studies could include evaluating the effects of hypothermia in a therapeutic range (32℃-34℃) and comparing the effects of repetitive hypothermic exposure with a single exposure on the brain.



 PDF Links
PDF Links PubReader
PubReader PubMed
PubMed Download Citation
Download Citation


