Introdction
Renal amyloidosis is characterized by the extracellular deposition of amyloid, a proteinaceous fibrilar material, presenting positive staining of Congo red, in the kidney1). Primary amyloidosis is extremely rare in children and the causes of secondary amyloidosis in children include familiar Mediterranean fever (FMF), rheumatoid arthritis (RA), ankylosing spondylitis, inflammatory bowel disease, chronic infection such as tuberculosis, and cystic fibrosis (CF)2). Bronchiectasis in children is most commonly associated with CF in industrialized countries, although there are many etiologies for bronchiectasis unrelated to CF3). Herein, we present a 13-year-old Korean girl who developed secondary renal amyloidosis by non-CF related bronchiectasis.
Case report
A 13-year-old girl was admitted with dyspnea due to severe pneumonia and respiratory failure. On past medical history, she developed bronchiolitis at the early age of 1 month for the first time. Since that time, pneumonia and bronchiolitis recurred several times a year up to school age. The medical history of her family was unremarkable. When she was 10-years-old, she was referred to our department for the evaluation of abnormal infiltration of both lung fields visible on a chest X-ray. At that time surgical removal of nasal polyps due to chronic sinusitis was decided and a chest X-ray was checked as one of preoperative evaluations. However, she had no respiratory symptoms or signs and her breathing sound on auscultation was clear. Several evaluations to find the causes of this abnormal infiltration were performed, especially by tuberculosis tests. But AFB stain, PCR and culture of sputum and urine were all negative and a Mantoux test was also negative. So, she was diagnosed with simple infectious bronchiolitis with bronchitis. After that, she was followed up irregularly during the next 3 years. Several months before admission, she had been also diagnosed with hypothyroidism and gastritis at another hospital.
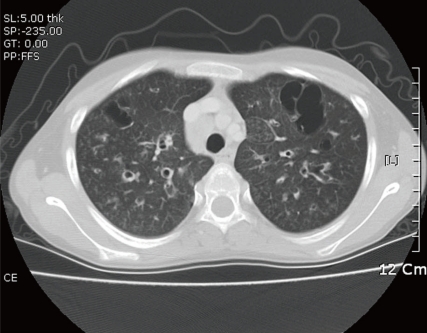
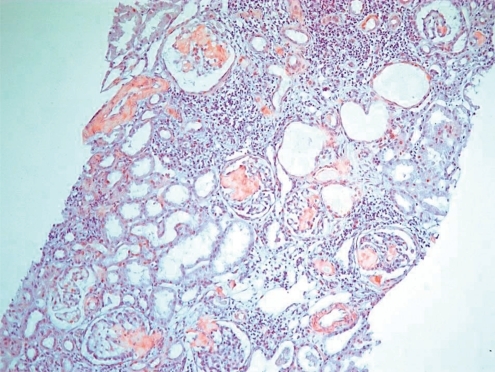
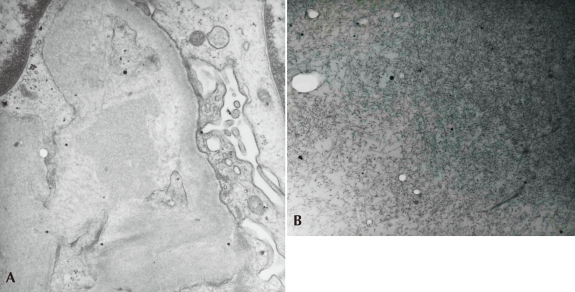
On physical examination, she had lip cyanosis and clubbing fingers. A computed tomography (CT) scan showed multifocal bronchiectasis and extensive bronchiolitis (Fig. 1). In the course of the admission, she was supported by mechanical ventilation for nearly one month and by the parenteral treatment of antibiotics for pseudomonas infection proven by culture of sputum. She has received chest physiotherapy to improve mucus clearance with mucolytic drug. Systemic and inhaled corticosteroids were also applied for short-term period. On admission, the laboratory findings of this girl were as follows: pH 7.37 and pCO2 35 mmHg on arterial blood gas analysis, white blood cell count 22,860/mm3, hemoglobin 9.3 g/dL, platelet count 757,000/mm3, erythrocyte sedimentation rate 83 mm/hour, C-reactive protein 11.18 mg/dL, blood urea nitrogen (BUN) 4.4 mg/dL, serum creatinine 0.42 mg/dL, total protein 4.8 g/dL, and albumin 1.8 g/dL. Cold agglutinin and antibody to mycoplasma were negative. Urinalysis showed protein (++), occult blood (+) and urine protein during 24 hours was 2,090 mg/m2. To elucidate the cause of bronchiectasis, several additional tests were also performed. Immunologic studies showed normal levels of immunoglobulin (Ig) G, IgA, IgM and α1-antitrypsin except for a mild elevation of IgE. Several weeks later a sweat test performed as one of the evaluations of CF was not specific and there was no mutation of the CF transmembrane conductance regulator (CFTR) gene. After confirming non-CF related bronchiectasis, a mucosal biopsy of the nasal cavity was performed to rule out primary ciliary dyskinesia and the result was normal on an electron microscopy (EM). Six months later after an improvement of her respiratory condition, an ultrasonogram-guided renal biopsy was performed to reveal the cause of proteinuria. On light microscopy, there were many segmental homogeneous deposits of amyloid with positive Congo red staining in the glomeruli and interstitium (Fig. 2). Immunohistochemistry revealed positive staining of the AA amyloid and immunofluorescent microscopy showed negative staining of IgG, IgA, IgM, C3 and fibrinogen. EM showed relatively straight, non-branching, randomly arranged amyloid fibrils in the mesangium of the glomeruli and these fibrils were approximately 10 nm in diameter (Fig. 3A, 3B), compatible with secondary amyloidosis. At that time, serum amyloid A was remarkably elevated (62.0 mg/L, normal range: <8.0 mg/L).
At recent follow-up (22 months after admission), BUN and serum creatinine were mildly elevated (34 mg/dL and 1.2 mg/dL, respectively) and proteinuria was improved with the results of the protein (+) in dipstick and decreased spot urine protein-creatinine ratio. The repeated chest film and CT scans showed no remarkable interval change and serum amyloid A slightly decreased than before (26.9 mg/L).
Discussion
Amyloidosis is a group of diseases characterized by extracellular deposition of insoluble fibrous amyloid proteins which can be identified in biopsy specimens both by their characteristic appearance on the EM and by their ability to bind Congo red. And the major sites of amyloid deposition are in kidney, heart, skin, gastrointestinal tract, and liver4). Clinically evident renal involvement mainly occurs in immunoglobulin light chains in primary systemic amyloidosis (AL) or amyloid A in secondary amyloidosis (AA)4). Because primary amyloidosis is extremely rare in children2), secondary amyloidosis is the major pattern of renal amyloidosis during childhood.
Causes of secondary amyloidosis in children include FMF, RA, ankylosing spondylitis, inflammatory bowel disease, chronic infections such as tuberculosis, CF2), and chronic granulomatous disease5). Although FMF is the most common cause of secondary amyloidosis in childhood6), FMF is highly prevalent in some limited areas such as the Middle East and Turkey1, 7). Tuberculosis and other chronic infections including bronchiectasis are other leading causes of systemic amyloidosis, followed in frequency by RA in other areas, especially developing countries1).
The clinical manifestations of renal amyloidosis vary with the site of involvement and most often includes asymptomatic proteinuria and nephrotic syndrome, as well as renal insufficiency1). End-stage renal disease (ESRD) is the cause of death in a minority of patients. Moon et al8) reported the first case of secondary renal amyloidosis in a 12-year-old girl with juvenile rheumatoid arthritis.
Conditions that predispose bronchiectasis in children include CF, ciliary dyskinesia, hypogammaglobulinemia, proximal airway narrowing, and Kartagener syndrome3, 9). Among these etiologies, CF is the most common cause of bronchiectasis in children, especially, in industrialized countries, although there are many etiologies for bronchiectasis unrelated to CF3). However, because CF is very rare in Korea, the incidence of bronchiectasis in Korean children is extremely low10). Lung damage occurring after lower respiratory infection is still the most common cause of bronchiectasis in developing countries and a great problem in some parts of the world11). In our patient, she had a history of recurrent respiratory infections such as pneumonia, bronchiolitis and bronchitis from infancy. Moreover, she was not found to have any other underlying etiology. Therefore, it is likely that an underlying etiology of the bronchiectasis in our patient is postinfectious.
Development and progression of secondary amyloidosis, in general, needs the long-term continuation of primary inflammatory conditions that induce the elevation of amyloid protein as one of the acute phase reactants. It was reported that the mean duration of bronchiectasis in patients with secondary amyloidosis to cause chronic renal failure was 22 years12). Therefore, it is very difficult to find patients with secondary renal amyloidosis complicated by long-standing, non-CF related bronchiectasis during childhood in Korea.
In the pathogenesis of secondary amyloidosis, the importance of increased serum amyloid A (SAA) has been demonstrated by the correlation between SAA concentration and disease course in patients with established amyloidosis13). The outcomes were variable but, among those in whom the level of SAA was persistently above 50 mg/L, the amyloid load usually increased and organ function deteriorated13). Actually, in our patient, her SAA was highly elevated (62.0 mg/L) and this confirmed the existence of uncontrolled systemic inflammation. If untreated, AA amyloidosis is a serious disease with a significant mortality due to ESRD, infection, heart failure, bowel perforation, or gastrointestinal bleeding. In a Japanese study of 42 patients with AA amyloidosis associated with rheumatoid arthritis the ten-year survival was 18 percents and was adversely impacted by the presence of azotemia and/or cardiac involvement at the time of diagnosis14).
Successful treatment of the underlying inflammatory process can lead to stabilization of or improvement in renal function, reduction in protein excretion, and partial resolution of amyloid deposit15). Therefore, the decision was made to perform more aggressive treatments to decrease the frequency of recurrent infection originating from bronchiectasis and it was believed that this treatment could ultimately put off the progression of renal amyloidosis. On the other hand, SAA levels in RA are increased in both patients with and without amyloidosis and this indicates that additional factors must be involved in pathogenesis16, 17).
In spite of aggressive evaluations, the cause of bronchiectasis in our patient was not revealed, but we could rule out CF and ciliary dyskinesia which are major causes of bronchiectasis by several evaluations such as sweat test, study of CFTR gene and mucosal biopsy on nasal cavity. To our knowledge, this report is the first case of secondary renal amyloidosis induced by non-CF related bronchiectasis in Korean children.



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link PubMed
PubMed Download Citation
Download Citation


