Article Contents
| Korean J Pediatr > Volume 53(9); 2010 |
Abstract
Purpose
Microalbuminuria is defined as increased urinary albumin excretion (30-300 mg/day) or microalbumin/creatinine ratio (30-300 mg/g) in a spot urine sample. Although microalbuminuria is a predictor of clinical nephropathy and cardiomyopathy, few studies have investigated microalbuminuria in children with urinary tract infection (UTI).
Methods
Therefore, we compared the spot urine microalbumin/creatinine ratio in pediatric UTI patients with that of control subjects. We investigated the correlation between the ratio in children with UTI and age, height, weight, blood pressure, glomerular filtration rate (GFR), hematuria, vesicoureteral reflux, renal parenchymal defect, and renal scar, and its predictability for UTI complications.
Results
We studied 66 patients (42 boys, 24 girls) and 52 healthy children (24 boys, 28 girls). The mean microalbumin/creatinine ratio in UTI patients was statistically significantly increased compared to the control group (340.04±321.36 mg/g (38.47±36.35 mg/mmol) in patient group vs. 225.68±154.61 mg/g (25.53±17.49 mg/mmol) in control group, P=0.0141). The mean value of spot urine microalbumin/creatinine ratio (384.70±342.22 mg/g (43.47±37.67 mg/mmol) in patient group vs. 264.92±158.13 mg/g (29.94±17.86 mg/mmol) in control group, P=0.0341) in 1-23 months age patient group showed statistically significant increase compared to control group. Microalbumin/creatinine ratio showed negative correlation to age (r=-0.29, P=0.0167), body surface area (BSA) (r=-0.29, P=0.0173) and GFR (r=-0.26, P=0.0343). The presence of hematuria (P=0.0169) was found to be correlated.
Conclusion
The spot urine microalbumin/creatinine ratio in children with UTI was significantly greater than that in normal children, and it was positively correlated with GFR. This ratio is a potential prescreening and prognostic marker in UTI patients. Further studies are required to validate the predictability of microalbuminuria in pediatric UTI patients.
Microalbuminuria is defined as increased urinary albumin excretion, between 30 and 300 mg/day or between 30 and 300 mg/g microalbumin/creatinine ratio in a spot urine sample1-3). It is known as a predictor of clinical nephropathy in patients with diabetes mellitus, and a risk factor of cardiomyopathy and nephropathy in the general adult population4). Few studies have investigated microalbuminuria in children and the validity of prescreening by measuring microalbuminin in spot urine samples to identify children with urinary tract infection (UTI)5, 6). We therefore elected to compare the spot urine microalbumin/creatinine ratios of pediatric patients with UTI (patient group) with that of healthy control subjects (control group). We assessed the correlation between spot urine microalbumin/creatinine ratio and age, height, weight, blood pressure, glomerular filtration rate (GFR), hematuria, vesicoureteral reflux (VUR), renal parenchymal defect and renal scar in children with UTI, and the predictability of microalbumin/creatinine ratios in spot urine samples for UTI complications.
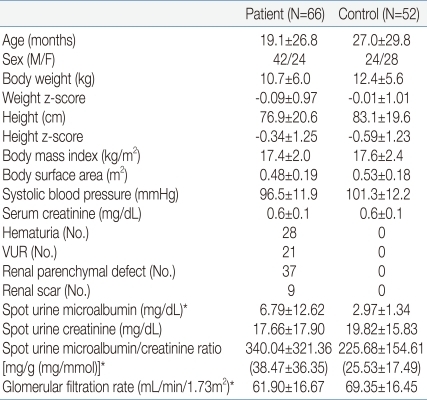
The age, height, weight, blood pressure, serum glucose, serum creatinine, spot urine creatinine and microalbumin in 66 (42 boys and 24 girls) pediatric patients with UTI admitted to the Konkuk University Hospital from July 2007 to September 2009, and 52 (24 boys and 28 girls) healthy children were evaluated. The patient group had fever, pyuria and fulfilled the diagnostic criteria of UTI: the presence of more than 100,000 colonies of a single pathogen in urinary culture7). Spot morning urine creatinine and microalbumin in UTI patients were evaluated when fever was subsided and follow-up urine culture was negative. Hematuria was defined 5 or more RBCs per high-power field (HPF) in specimen or a positive reaction on the urine dipstick test8). Persistent hematuria or glomerulonephropathy were excluded in this study. VUR or renal parenchymal defects were detected by voiding cystoureterography (VCUG) and 99mTc-dimercaptosuccinicacid (DMSA) scan. VUR was graded on a scale of I to V according to the classification defined by the international reflux grading system9). Age, sex, height z-score, weight z-score were matched between the patient and control groups. Height z-score, weight z-score were determined using the LMS method. GFR, adjusted for body surface area was calculated using the Schwartz formula [GFR (mL/min/1.73m2)=κ×height (cm)/serum creatinine (mg/dL), κ=0.33 for low birth weight infants, 0.45 for term appropriate gestation age infants, 0.55 for children from 2-13 years and adolescent girls and 0.70 for adolescent boys]8). Spot urine microalbumin/creatinine ratio was expressed in SI units of mg/g and mg/mmol. Conversion to mg/mmol was calculated using the following formula: microalbumin/creatinine ratio (mg/mmol)=urine microalbumin (mg/L)×1,000/urine creatinine (mg/dL)×88.43. Statistical analysis was performed on log-transformed data, since the spot urine microalbumin/creatinine ratio approximates a log-normal distribution. Differences in mean values were evaluated by t-test and one-way ANOVA test. Comparisons between spot urine microalbumin/creatinine ratio and other variables were made using Pearson's correlation analysis. A P-value of less than 0.05 was considered to be statistically significant.
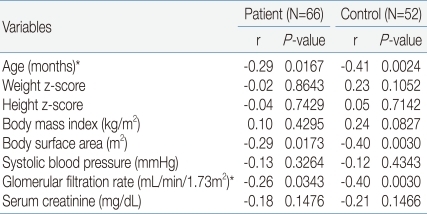
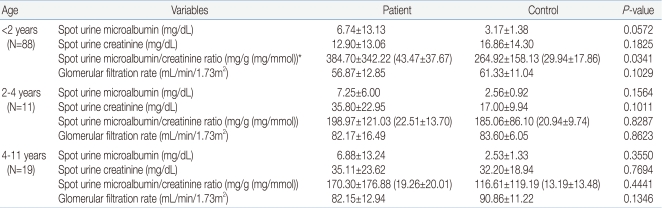
We studied 66 UTI patients (42 boys, 24 girls) and 52 normal children (24 boys, 28 girls). Ages were 19.1±26.8, 27.0±29.8 months respectively, ranging 1 month to 11 years old. In 28 children there was transient microscopic hematuria. Twenty one of UTI patients had VUR, 37 had renal parenchymal defects, 9 had renal scar. Anthropometric, laboratory and radiologic data are given in Table 1. The mean value of spot urine microalbumin (6.79±12.62 mg/dL in patient group vs. 2.97±1.34 mg/dL in control group, P=0.0172), microalbumin/creatinine ratio in patient group showed a statistically significant increase compared to control group [340.04±321.36 mg/g (38.47±36.35 mg/mmol) in patient group vs. 225.68±154.61 mg/g (25.53±17.49 mg/mmol) in control group, P=0.0141]. GFR in the patient group was statistically significantly decreased in comparison to the control group (61.90±16.67 mL/min/1.72m2 in patient group vs. 69.35±16.45 mL/min/1.72m2 in control group, P=0.0168) (Table 3). Age (r=-0.29, P=0.0167 in patient group, r=-0.41, P=0.0024 in control group), body surface area (BSA) (r=-0.29, P=0.0173 in patient group, r=-0.40, P=0.0030 in control group), GFR (r=-0.26, P =0.0343 in patient group, r=-0.40, P =0.0030 in control group) were found to be negatively correlated to spot urine microalbumin/creatinine ratio (Table 2, Fig. 1A, 1B). The mean value of spot urine microalbumin/creatinine ratio in 1-23 months age patient group showed a statistically significant increase compared to control group (384.70±342.22 mg/g (43.47±37.67 mg/mmol) in patient group vs. 264.92±158.13 mg/g (29.94±17.86 mg/mmol) in control group, P=0.0341). GFR in 1-23 months age patient group showed a decrease compared to control group (56.87±12.85 mL/min/1.72m2 in patient group vs 61.33±11.04 mL/min/1.72m2 in control group, P=0.1029), but it was no statistical significance (Table 3). In a subset of 13 children with follow-up, mean spot urine microalbumin/creatinine ratio was statistically decreased (565.51±583.74 mg/g to 192.77±275.20 mg/g, P =0.0481). Presence of transient microscopic hematuria (P=0.0169) was found to be correlated. VUR, VUR grade, renal parenchymal defect and renal scar were not correlated to spot urine microalbumin/creatinine ratio.
Although UTI is a common disease in children and is said to be associated with proteinuria or microalbuminuria, the relationship between microalbuminuria and UTI remains incompletely defined11). Brocklebank et al.12) demonstrated a mixed tubular/glomerular pattern of proteinuria in patients with ureteral reflux. There exist studies that utilized specific quantitative immunoassays to demonstrate tubular proteinuria patterns in patients with pyelonephritis13). It was well known that epithelial cells lining the proximal tubule reabsorbed filtered albumin using receptor-mediated endocytosis. Microalbuminuria results when the filtered load of albumin exceeds tubular re-absorptive capacity14, 15). In glomerular injury, the filter became more 'leaky' and allowed greater amounts to filter through, overwhelming the tubular reabsorption mechanism in the meantime16). It was thought that patients with severe pyelonephritis or renal scar could have had compensatory hyperfiltration in the remaining nephrons and developed progressive glomerulosclerosis, as evidenced by increased urine microalbumin excretion13, 17). A recent report suggested that microalbuminuria might promote tubular injury with interstitial inflammation and eventual fibrosis18). Khatib et al.19) found focal segmental glomerulosclerosis in adult patients with renal scarring, and good correlation was observed between the presence and extent of focal glomerulosclerosis, and the degree of proteinuria. Therefore it could be said that early microalbuminuria in patients with UTI is an indicator of high permeability of glomerular basement membrane (GBM) and disease progression.
Few study investigated spot urine microalbumin/creatinine ratio in children5, 6). Several prior reports indicated that spot urine samples provide a good estimate of the 24-hour excretion rate20, 21). However, 24-hour timed urine collection is hard to obtain in pediatric patients, whereas spot urine microalbumin/creatinine ratio represents a simple and reliable clinical marker for early identification of renal injury in children with UTI22, 23).
In this study, the level of microalbumin and the spot urine microalbumin/creatinine ratio were higher in the patient group compared to control, and the absolute number of children with microalbuminuria was large (N=112). Spot urine microalbumin/creatinine ratio is simple to obtain, but the result may incorrectly suggest elevated microalbumin excretion rate simply because the patient has low creatinine excretion due to reduced muscle mass, particularly in women and infants15, 24). Most children included in this study were less than 2 years-old, therefore the spot urine creatinine and microalbumin/creatinine ratio of children might be increased compared to that of adults. Because the children included in this study were younger than other reported studies, the prevalence of microalbuminuria is high. It was observed that mean spot urine microalbumin/creatinine ratio for 1-23 months was 384.70±342.22 mg/g (43.47±37.67 mmol/mg) dropping to 170.30±176.88 mg/g (19.26±20.01 mmol/mg) for 4-11 years age group. It could be due to a decreased in the amount of filtered albumin or to an increased in tubular reabsorption and catabolism following maturation of the proximal tubules with age25). In a subset of 13 children with follow-up, the value was statistically decreased. Kim et al.26) reported elevated albuminuria is largely transient in nondiabetic youth, but persistent and progressive in those with diabetes. In this study, as most of the patient group had short disease duration and no permanent renal disease, microalbuminuria in UTI patient group is transient. With further study, it might be used as an early detector and a follow-up marker in UTI patients.
In patient and control groups, BSA and GFR were negatively correlated to spot urine microalbumin creatinine ratio. Karlen et al.13) reported GFR was negatively correlated to urine microalbumin excretion in patients with pyelonephritic renal injuries, and Keijzer-Veen et al.27) reported 19-year-old subjects born very premature or intrauterine growth retardation (IUGR) had low GFR and microalbuminuria. Birth weight was associated negatively with microalbumin/creatinine ratio and positively with GFR.
A limitation of this study is that only one spot urine microalbumin/creatinine ratio was obtained, and it was not compared to timed urine collections. Yoon et al.5) reported that the spot urine microalbumin/creatinine ratio was significantly increased in children with VUR and renal defects. Basic et al.18) determined the presence of microalbuminuria and decreased creatinine clearancerate (CCR) in children with high grade of VUR. In this study VUR, VUR grade, renal parenchymal defect and renal scar were not correlated to microalbuminuria. The discrepancy between the findings reported in our study and reported by Yoon et al.5) is likely due to difference of mean age of patients, severity of disease. Therefore further longitudinal follow-ups are required to confirm the apparent absence of correlation.
In conclusion, the spot urine microalbumin/creatinine ratio in children with UTI was statistically significantly increased compared to that of normal children. In addition, the spot urine microalbumin/creatinine ratio was related to GFR. The ratio could potentially be used as a pre-screening and prognostic marker in UTI patients. Further studies are required to validate the predictability of microalbuminuria in pediatric UTI patients.
References
2. Gerstein HC, Mann JF, Yi Q, Zinman B, Dinneen SF, Hoogwerf B, et al. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA 2001;286:421–426.


3. Burgert TS, Dziura J, Yeckel C, Taksali SE, Weiss R, Tamborlane W, et al. Microalbuminuria in pediatric obesity: prevalence and relation to other cardiovascular risk factors. Int J Obes (Lond) 2006;30:273–280.


5. Yoon JR, Koo JW. Urinary protein and enzyme excretion of spot urine in children with vesicoureteral reflux. J Korean Soc Pediatr Nephrol 2009;13:56–62.

6. Chiou YY, Chiu NT, Chen MJ, Cheng HL. Role of beta 2-microglobulinuria and microalbuminuria in pediatric febrile urinary tract infection. Acta Paediatr Taiwan 2001;42:84–89.

7. Elder JS. Kliegman RM,Urinary tract infections. editor. Nelson textbook of pediatrics. 2006;18th ed. Philadelphia: WB Saunders Co, :2223–2228.
8. Assadi FK. Value of urinary excretion of microalbumin in predicting glomerular lesions in children with isolated microscopic hematuria. Pediatr Nephrol 2005;20:1131–1135.


9. Popovie-Rolovic M, Peco-Antic A, Marsenic O. Popovie-Rolovic M, Peco-Antic A, Marsenic O,Vesicoureteral reflux and reflux nephropathy. editors. Pediatric nephrology. 2001;Belgrade, Serbia: Nauka, :68–77.
10. Schwartz GJ, Brion LP, Spitzer A. The use of plasma creatinine concentration for estimating glomerular filtration rate in infants, children, and adolescents. Pediatr Clin North Am 1987;34:571–590.


11. Carter JL, Tomson CRV, Stevens PE, Lamb EJ. Does urinary tract infection cause proteinuria or microalbuminuria? A systematic review. Nephrol Dial Transplant 2006;21:3031–3037.


12. Brocklebank T, Cooper EH, Richmond K. Sodium dodecyl sulphate polyacrylamide gel electrophoresis patterns of proteinuria in various renal diseases of childhood. Pediatr Nephrol 1991;5:371–375.


13. Karlen J, Linne T, Wikstad I, Aperia A. Incidence of microalbuminuria in children with pyelonephritic scarring. Pediatr Nephrol 1996;10:705–708.


14. Tomlinson PA, Smellie JM, Prescod N, Dalton RN, Chantler C. Differential excretion of urinary proteins in children with vesicoureteric reflux and reflux nephropathy. Pediatr Nephrol 1994;8:21–25.


15. Bakris GL. Microalbuminuria: prognostic implications. Curr Opin Nephrol Hypertens 1996;5:219–223.


17. Brunskill NJ. Molecular interactions between albumin and proximal tubular cells. Exp Nephrol 1998;6:491–495.


18. Basic J, Golubovic E, Miljkovie P, Bjelakovic G, Cvetkovic T, Milosevic V. Microalbuminuria in children with vesicourethral reflux. Ren Fail 2008;30:639–643.


19. El-Khatib MT, Becker GJ, Kincaid-Smith PS. Morphometric aspects of reflux nephropathy. Kidney Int 1987;32:261–266.


20. Cowell CT, Rogers S, Silink M. First morning urinary albumin concentration is a good predictor of 24-hour urinary albumin excretion in children with type 1 (insulin-dependent) diabetes. Diabetologia 1986;29:97–99.


21. Bakker AJ. Detection of microalbuminuria. Receiver operation characteristic curve analysis favors albumin-to-creatinine ratio over albumin concentration. Diabetes Care 1999;22:307–313.


22. Keane WF, Eknoyan G. Proteinuria, albuminuria, risk, assessment, detection, elimination (PARADE): a position paper of the National Kidney Foundation. Am J Kidney Dis 1999;33:1004–1010.


23. Elises JS, Griffiths PD, Hocking MD, Taylor CM, White RH. Simplified quantification of urinary protein excretion in children. Clin Nephrol 1988;30:225–229.

24. De Jong PE, Brenner BM. From secondary to primary prevention of progressive renal disease: the case for screening for albuminuria. Kidney Int 2004;66:2109–2118.


25. Siegal SR, Oh W. Renal function as a marker of human fetal maturation. Acta Paediatr Scand 1976;65:481–485.

Fig. 1
(A) Correlation of age and microalbumin/creatinine ratio in the urinary tract infection (UTI) patient group. (B) Correlation of glomerular filtration rate (GFR) and microalbumin/creatinine ratio in the UTI group.




 PDF Links
PDF Links PubReader
PubReader PubMed
PubMed Download Citation
Download Citation


