Article Contents
| Korean J Pediatr > Volume 53(11); 2010 |
Abstract
Juvenile idiopathic arthritis (JIA) is comprised of a heterogeneous group of several disease subtypes that are characterized by the onset of arthritis before the age of 16 years and has symptoms lasting at least 6 weeks. The previous classification of JIA included seven different categories, whereas its current classification was compiled by the International League of the Association for Rheumatology, and replaced the previous terms of "juvenile chronic arthritis" and "juvenile rheumatoid arthritis," which were used in Europe or North America, respectively, with the single nomenclature of JIA. As mentioned above, JIA is defined as arthritis of unknown etiology that manifests itself before the age of 16 years and persists for at least 6 weeks, while excluding other known conditions. The clinical symptoms of JIA can be quite variable. Several symptoms that are characteristic of arthritis are not necessarily diagnostic of JIA and may have multiple etiologies that can be differentiated with careful examination of patient history. The disease may develop over days or sometimes weeks, thereby making the diagnosis difficult at the time of presentation. To make a clinical diagnosis of JIA, the first step is to exclude arthritis with known etiologies. Of note, late treatment due to excessive delay of diagnosis can cause severe damage to joints and other organs and impair skeletal maturation. Therefore, early detection of JIA is critical to ensure prompt treatment and to prevent long-term complications including the likelihood of disability in childhood.
Juvenile idiopathic arthritis (JIA) is comprised of a heterogeneous group of several disease subtypes that are characterized by the onset of arthritis prior to the age of 16 years with symptoms that persist for more than 6 weeks. The occurrence of chronic arthritis in children has been described since the 1800s. George Frederick Still, an English pediatrician, described 22 children from Hospital for Sick Children, Great Ormond Street that had chronic arthritis1). It is still believed that 12 of these children had distinctive features of rheumatoid arthritis. Since this report, many additional children have been described with rheumatoid arthritis, and it has become obvious that the disease we now call JIA or juvenile rheumatoid arthritis (JRA) is a particularly unpredictable and heterogeneous disease group. The previous classification of JIA described 7 categories of the disease2), and the currently used classification was compiled by the International League of Associations for Rheumatology (ILAR). According to the updated ILAR system, the previous terms of "juvenile chronic arthritis (JCA)" and "JRA," which are used in Europe or North America, respectively, are replaced by the collective term JIA. Moreover, as mentioned above, this system defines JIA as arthritis of unknown etiology beginning before the age of 16 years and persisting for at least 6 weeks, while excluding other known conditions. As a consequence of the definition, JIA is not diagnosed before 6 weeks of onset, and final classification into one of its 7 subgroups is typically performed after 6 months2).
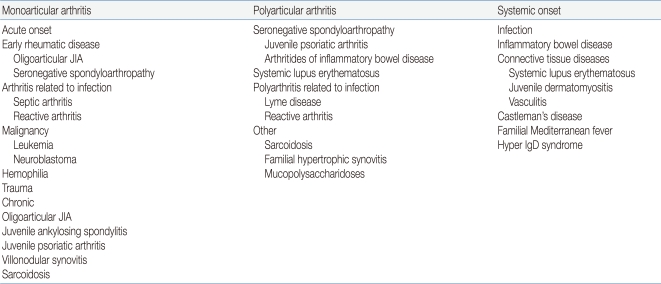
The classification of JIA provides an important framework for its research, as well as for assisting with identification of appropriate treatment and prediction of natural disease history. The aim of the ILAR classification system of JIA is to explain relatively homogeneous, mutually exclusive categories of idiopathic childhood arthritis based on predominant clinical laboratory features2). The current categories of JIA are shown in Table 12).
The clinical symptoms of JIA can be variable. Several symptoms that indicate arthritis are not necessarily diagnostic of JIA, and may have multiple etiologies that can be differentiated with careful examination of patient history3). Polyarticular joint disease has a multi-factorial etiology, and may present as a viral illness or can be the beginning of a chronic disease. Moreover, its underlying etiological process can be infectious or post-infectious with a rheumatological disease or a manifestation of systemic disease. This disease may evolve over days or sometimes weeks, thereby making the diagnosis difficult at the time of presentation4). Thus, to accurately diagnose JIA, the first step is to exclude arthritis with known etiologies4) (Table 2).
In a child with acute-onset monoarthritis, the differential diagnosis must include septic arthritis, trauma, and hematologic diseases5). A painful joint effusion of short duration may be caused by trauma. It has been suggested that JIA may be the most common cause of chronic oligoarthritis. In a child with oligoarticular JIA, the affected joint is swollen and often warm, but is not typically very painful, tender, or red5). Moreover, if a joint is extremely painful and erythematous, or if the child is febrile, septic arthritis is more likely the correct diagnosis6). Such patients should undergo a test of prompt joint aspiration to exclude septic arthritis and osteomyelitis. The onset of polyarthritis in a preadolescent or adolescent girl potentially suggests the diagnosis of systemic lupus erythemtosus (SLE). The arthritis of SLE may follow that of JIA, but without undertaking serologic studies, its correct diagnosis may be difficult to make until the detection of the more characteristic clinical symptoms of SLE.
The differential diagnosis of a child suspected of having systemic JIA is often difficult, especially at the onset or early in the course of the disease, when the child may have a high spiking fever with evidence of systemic inflammation but no arthritis or other specific signs that allow for a definitive diagnosis7). Children with systemic JIA may first be thought to have an acute infectious disease or septicemia. However, the presence of arthritis and/or a rheumatoid rash helps to establish an accurate diagnosis of systemic JIA. In contrast, laboratory tests may be of little value in diagnosing systemic JIA. Fever in children with infectious diseases is of the septic type; this type of fever is clinically more confusing and typically does not repetitively return to the baseline each day, as does the fever associated with JIA. That is, the diagnostic symptom of systemic JIA is a high spiking fever8), which may occur at any time of the day but is commonly present in the late afternoon to evening in conjunction with rash. In contrast, the temperature of an individual affected by JIA may be below normal in the morning. Moreover, JIA-associated fever can frequently accompany chills. Children commonly appear unwell while febrile, but surprisingly well during the rest of the day.
In our experience of assessing patients suspected of having JIA from 1990 to 2000, 51 cases were excluded to other diagnoses, which included arthritis related to infection (61%), malignancy (6%), other rheumatological disorders (26%), and immunodeficiency syndrome (4%)9).
As briefly mentioned above, the diagnosis of JIA is essentially a clinical one. No laboratory test or combination of studies can confirm the diagnosis of JIA. However, the laboratory studies can be used to provide evidence of inflammation, support the clinical diagnosis of JIA, monitor toxicity of therapy, and better understand the pathogenesis of the disease.
Hematologic abnormalities generally reflect the extent of the inflammatory disease. Useful laboratory investigations include count of blood cells (CBC) and inflammatory markers like erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP). However, the levels of ESR and CRP in active JIA are variable. In general, ESR is a useful measure of active disease at onset and during follow-up visits with a child with JIA, especially in polyarticular or systemic onset. Also, ESR is occasionally helpful in monitoring therapeutic efficacy. A CBC may show anemia of chronic disease if there has been longstanding inflammation. JIA-associated anemia is attributable to chronic disease (low serum iron, low total iron-binding capacity, adequate hemosiderin store), although iron deficiency may also play an important role. It has been established that plasma iron transport and the amount of iron available for erythropoiesis decreases in individuals with systemic disease. Leukocytosis is common in children with active disease, and their platelet count may rise dramatically in cases with severe systemic or polyarticular involvement5). The concentrations of the serum immunoglobulins, IgG, IgA, and IgM, were previously determined in 200 patients with juvenile rheumatoid arthritis, which showed abnormality in the distribution of these immunoglobulins in JIA10). The serum levels of immunoglobulins are reportedly correlated with the activity of the disease and the acute phase response10, 11).
In a study of the diagnostic utility of rheumatoid factor (RF) serology in children, RF tests were shown to likely be positive in children with both JIA and diseases other than JIA12). Thus, evaluation of RFs is not useful as a diagnostic tool for JIA. However, children with high titers of RFs likely represent a subgroup distinctive from the larger number of children with seronegative disease. A RF test should be performed in patients with polyarthritis, as its presence has prognostic significance13). The presence of anti-nuclear antibody (ANA) deliberates risk for the development of asymptomatic uveitis, particularly in those individuals with oligoarticular onset disease, although assessment of ANA should be performed in all JIA patients14, 15). Anti-cyclic citrullinated peptide (anti-CCP) antibodies are not routinely tested in JIA patients, but may indicate severe disease16). Human leukocytic antigen (HLA) B27 should be examined in children who present with symptoms of enthesitis-related arthritis and can indicate susceptibility to the development of axial arthritis. Furthermore, if there is concern that arthritis is part of an underlying connective tissue disease or vaculitis, then it may be useful to test dsDNA, extractable nuclear antigen (ENA), C3, C4, and immunoglobulin levels. Finally, as most of the rheumatological tests lack in desired specificity, results should always be interpreted in the context of a given patient4, 17, 18).
Conventional radiographs are the most easily available, quick, and inexpensive method of evaluating a joint. Ultrasonography is often the best way of identifying intra-articular fluid, particularly in joints such as the hip and shoulder, where fluid may be difficult to demonstrate clinically19). Increasing use of standard magnetic resonance (MR) imaging and the advances in more functional MR techniques over the past decade have improved the assessment of joint disease in JIA. Compared to ultrasonography or plain radiography, MR imaging is powerful in detecting inflammatory changes in the joint and cartilage damage. MR imaging is also able to precisely evaluate the later manifestations of JIA, including erosions, loss of joint space, cartilage damage, and ligamentous involvement19, 20) (Fig. 1). Moreover, bone scans using technetium-99m are also useful for detecting the early stage of inflammatory arthritis21) (Fig. 2). Unfortunately, no single modality meets every imaging requirement at this time, but methods for detecting JIA are dramatically improving22).
JIA is the most common rheumatic disease that affects children and is a significant cause of both short- and long-term disabilities. Specifically, JIA is defined as arthritis of unknown etiology and its diagnosis requires clinical exclusion of other known conditions. Excessive delay in instituting advanced treatment for JIA can result in irretrievable damage to joints and other organs and impair skeletal maturation. Thus, early detection of JIA is critical to ensure its prompt treatment and to prevent long-term complications, including the likelihood of disability during childhood.
References
1. Still GF. On a form of chronic joint disease in children. 1896. Clin Orthop Relat Res 1990;4–10.
2. Petty RE, Southwood TR, Manners P, Baum J, Glass DN, Goldenberg J, et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol 2004;31:390–392.

5. Cassidy JT, Petty RE. Textbook of Pediatric Rheumatology. 2001;4th ed. Phliladelphia: W.B Saunders, :258
6. Fink CW, Dich VQ, Howard J Jr, Nelson JD. Infections of bones and joints in children. Arthritis Rheum 1977;20:578–583.

7. Miller LC, Sisson BA, Tucker LB, Schaller JG. Prolonged fevers of unknown origin in children: patterns of presentation and outcome. J Pediatr 1996;129:419–423.


8. McMinn FJ, Bywaters EG. Differences between the fever of Still's disease and that of rheumatic fever. Ann Rheum Dis 1959;18:293–297.



10. Cassidy JT, Petty RE, Sullivan DB. Abnormalities in the distribution of serum immunoglobulin concentrations in juvenile rheumatoid arthritis. J Clin Invest 1973;52:1931–1936.



11. Hoyeraal HM, Mellbye OJ. Humoral immunity in juvenille rheumatoid arthritis. Ann Rheum Dis 1974;33:248–254.



12. Eichenfield AH, Athreya BH, Doughty RA, Cebul RD. Utility of rheumatoid factor in the diagnosis of juvenile rheumatoid arthritis. Pediatrics 1986;78:480–484.



13. Shin YS, Choi JH, Nahm DH, Park HS, Cho JH, Suh CH. Rheumatoid factor is a marker of disease severity in Korean rheumatoid arthritis. Yonsei Med J 2005;46:464–470.



14. Petty RE, Smith JR, Rosenbaum JT. Arthritis and uveitis in children. A pediatric rheumatology perspective. Am J Ophthalmol 2003;135:879–884.


15. Shin JI, Kim KH, Chun JK, Lee TJ, Kim KJ, Kim HS, et al. Prevalence and patterns of anti-nuclear antibodies in Korean children with juvenile idiopathic arthritis according to ILAR criteria. Scand J Rheumatol 2008;37:348–351.


16. Kuna AT, Lamot L, Miler M, Harjacek M, Simundic AM, Vrkic N. Antibodies to mutated citrullinated vimentin and antibodies to cyclic citrullinated peptides in juvenile idiopathic arthritis. Clin Chem Lab Med 2009;47:1525–1530.

17. Kunnamo I, Kallio P, Pelkonen P, Hovi T. Clinical signs and laboratory tests in the differential diagnosis of arthritis in children. Am J Dis Child 1987;141:34–40.


18. Aeschlimann A, Schlumpf U. [Laboratory diagnosis of monarthritis: how much, what for, when?]. Schweiz Rundsch Med Prax 1993;82:419–427.

19. Fedrizzi MS, Ronchezel MV, Hilario MO, Lederman HM, Sawaya S, Goldenberg J, et al. Ultrasonography in the early diagnosis of hip joint involvement in juvenile rheumatoid arthritis. J Rheumatol 1997;24:1820–1825.

20. Lamer S, Sebag GH. MRI and ultrasound in children with juvenile chronic arthritis. Eur J Radiol 2000;33:85–93.


Fig. 1
Magnetic resonance imaging of a patient with juvenile idiopathic arthritis shows synovial proliferation and effusion collection. Thick prominent enhancement along the synovial lining of the knee joint is shown.
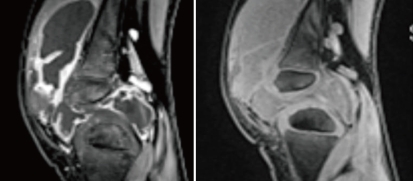
Fig. 2
Whole body bone scan using technetium-99m shows increased bone uptake in the lesions of a patient with juvenile idiopathic arthritis.
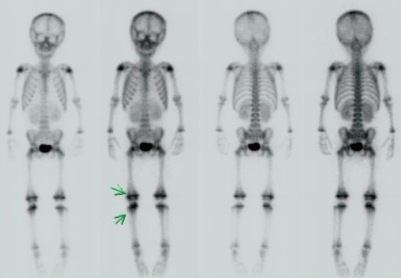
Table 1
International League of Associations for Rheumatology (ILAR) Classification of Juvenile Idiopathic Arthritis (JIA)2)

One of the major aims of the ILAR classification is the mutual exclusivity of the subtypes. Therefore, the following list of possible exclusion for each category was defined:
a) Psoriasis or a history of psoriasis in the patient or first-degree relative.
b) Arthritis in an HLA-B27-positive male beginning after the sixth birthday.
c) Ankylosing spondylitis, enthesitis-related arhtiris, sacroiliitis with inflammatory bowel disease or acute anterior uveitis or a history of one of these disorders in a first-degree relative.
d) The presence of IgM rheumatoid factor and at least two occasions at least 3 months a part.
e) The presence of systemic JIA in the patient
RF, Rheumatoid factor



 PDF Links
PDF Links PubReader
PubReader PubMed
PubMed Download Citation
Download Citation


