Article Contents
| Korean J Pediatr > Volume 54(5); 2011 |
Abstract
Purpose
The diagnosis of acute pyelonephritis (APN) is often difficult, as its clinical and biological manifestations are non-specific in children. If not treated quickly and adequately, however, APN may cause irreversible renal damage, possibly leading to hypertension and chronic renal failure. We were suspecting the diagnostic value of 99mTc-dimercaptosuccinic acid (DMSA) scan by experiences and so compared the results of DMSA scan to those of multi-detector row computed tomography (MDCT).
Methods
We retrospectively selected and analyzed 81 patients who were diagnosed as APN by MDCT during evaluation of their acute abdomen in emergency room and then received DMSA scan also for the diagnostic work-up of APN after admission. We evaluated the results of imaging studies and compared the diagnostic value of each method by age groups, <2 years (n=45) and ≥2 years (n=36).
Results
Among total 81 patients with MDCT-proven APN. DMSA scan was diagnostic only in 55 children (68%), while the remaining 26 children (32%) showed false negative normal findings. These 26 patients were predominantly male with average age of 21 months and most of them, 19 (73.1%) were <2 years of age.
Acute pyelonephritis (APN) is a suppurative inflammation of the renal interstitial tissue and pyelocaliceal lining. It is often multifocal, exhibiting different degrees of inflammation1). The early diagnosis is important in children, because children with APN frequently present with no specific symptoms; however, if not treated quickly and adequately, APN may cause irreversible renal damage, possibly leading to hypertension and chronic renal failure2,3). Certain clinical and biochemical findings, including flank pain; fever and chills; and increased white blood cell (WBC) count, erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP), are thought to indicate upper urinary tract infection (UTI), and one study has proposed that APN was associated with longer duration of fever prior to admission, longer duration of fever after beginning treatment, higher CRP concentration, and higher granulocyte count, with CRP concentration compared to lower UTI, having the best sensitivity and specificity4). Nevertheless, clinical signs alone in children who present with febrile UTI cannot predict the extent of renal damages, mild or multiple scarring5).
At present, ultrasonography, computer tomography (CT), and 99mTc-dimercaptosuccinic acid (99mTc-DMSA) scintigraphy are the most frequently used methods to image patients with suspected complications of UTI1). Magnetic resonance imaging is not practical because of the high cost and the need for continuous sedation due to longer time required for imaging. The recent development of power Doppler ultrasonography (PDU) may provide an additional method of detecting APN. Although PDU exposes patients to less radiation than CT or 99mTc-DMSA, its higher false-negative results and underestimation of the number of pyelonephritic lesions prevents its replacement of 99mTc-DMSA6).
To date, 99mTc-DMSA is regarded as the gold standard method of diagnosing APN. CT was recently shown to be more accurate than 99mTc-DMSA in detecting APN lesions in adult patients7) and 99mTc-DMSA scans were limited in identifying renal cortical lesions detected by CT in pediatric APN patients8). Moreover, new generation CTs showing excellent resolution compared with conventional CT, and improved quality of contrast media have made CT more practical.
Early diagnosis of APN in young children <2 years is particularly important because these children are at high risk for renal scarring9,10). Early diagnosis and treatment of APN can prevent or diminish renal scarring10-12). Diagnosis in children <2 years old is particularly difficult, because the clinical presentations are not typical. Neonates and infants with APN commonly present with non-specific symptoms such as irritability, poor feeding, jaundice, lethargy, vomiting, and diarrhea. We often experienced diagnostic limitations of DMSA scan, while working-up children with acute abdomen so we tried to compare the results of DMSA scan to MDCT.
Retrospectively, we selected and evaluated 81 patients who were diagnosed as APN by MDCT taken during evaluation of their acute abdomen in emergency room and also received DMSA scan for the diagnostic work-up of APN after admission to Ajou University Hospital from July 2003 to December 2009. All patients fulfilled the following inclusion criteria for the diagnosis of APN: fever (≥37.8℃, pyuria, and positive urine culture, except in pre-treatment cases with antibiotics.
Patients were excluded if they had experienced APN before. We evaluated the results of imaging studies and compared the diagnostic value of each method by age groups, <2 years (n=45) and ≥2 years (n=36).
All patients received DMSA scan within 5 days after the MDCT examination at emergency room. DMSA scans were performed with intravenous injection of weight-adjusted adult-equivalent dose of 37-MBq technetium-99 DMSA and single-photon emission computed tomography acquisition was performed for 20 minutes, approximately 4 hours after the isotope administration. Outcome of DMSA scan was considered positive if any one of the following condition was present: 1) change in the relative size of functioning renal mass, 2) an area of either decreased or absent activity within the cortex, or 3) a cortical defect extending to the collecting system.
MDCT scanning included non-enhancement examinations and the subsequent enhancement study, 3 minutes after the injection of contrast media for the nephrographic phase. Criteria for diagnosis of APN was composed of followings: 1) triangular zones of low attenuation with cortical basis, 2) streaky images corresponding to tubulointerstitial inflammation, and 3) round cortical areas. For the easy comparison, coronal reformed images of MDCT were obtained and compared with those of DMSA scintigraphy from the prospective of presence of lesions, laterality, location, number of lesions, and size of lesions.
To identify the characteristics of 81 patients with APN, descriptive statistics were obtained. Chi-square test was applied to compare the data between the two age groups, and independent variable t-test and chi-square test was taken to compare the clinical indicators of patients. SPSS ver. 12.0 (SPSS Inc., Chicago, IL, USA) was used for the statistical analysis and the significance level of less than 0.05 was considered statistically significant.
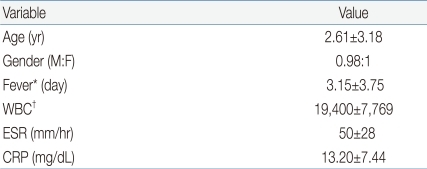
The age of 81 patients was 2.61±3.18 years and 40 were boys and 41 were girls, making the M:F ratio of 0.98:1. The duration of fever prior to hospitalization was 3.15 days. At the time of initial presentation of patients, peripheral blood WBC count was 19,400±7,769/mL; ESR was 50±28 mm/hr; and CRP concentration was 13.20±7.44 mg/dL (Table 1).
Among total 81 MDCT-proven cases with APN, the results of DMSA were positive or coincident only in 55 cases (68%). The remaining 26 cases (32%) showed normal or non-remarkable findings of DMSA, which was classified as 'false negative DMSA' based on the MDCT findings. These patients showed male predominance (1.6:1) and average age of 21 months. Most of them (19 patients, 73.1%) were under 2 years of age. The mean duration of febrile days before hospitalization was 1.96 days (Table 2).
When analyzing the age relationship to the false negative results of DMSA scan, 26 patients (57.8%) showed corresponding positive results and 19 patients (42.2%) showed false negative findings respectively, among 45 patients of aged <2 years old. Whereas, 29 (80.6%) had renal scan findings compatible to MDCT, 7 (19.4%) showed no remarkable findings among 36 patients aged ≥2 years. The rate of false negative DMSA scan was higher in younger children less than 2 years of age (P=0.029) (Fig. 1).
When the clinical and biochemical characters were compared to each other between the DMSA (-) group (n=26), showing non-correlating outcomes of DMSA scan to MDCT results and the DMSA (+) group (n=55), having consistent renal scan findings to MDCT, there was no difference in patient age, gender, and laboratory results. The amount of leukocytosis and increase in ESR and CRP concentration were not functioning as to telling the different outcomes of DMSA scan (Table 3).
When analyzing the anatomical pictures of renal lesions of MDCT in 26 cases of DMSA (-) group, 11 (42.3%) showed single focal lesions while 15 (57.7%) had multi-focal. In 12 cases (46.2%), lesions were limited to unilateral kidney and 12 (53.8%) had lesions in both kidney. There were no definite relationships found between erratic results of DMSA scan and the number or laterality of kidney lesions (Table 4).
An example of coronal reformation image of MDCT in 8-month-old boy shows the discrepancy in the results of imaging studies. 99mTc-DMSA scintigraphy failed to depict the lesions those were obvious on MDCT examination (Fig. 2).
The diagnosis of APN in children is often difficult, as the clinical and biological manifestations associated with this disease are non-specific. The classical features of flank pain, fever and chills, and increases in WBC counts, ESR and CRP concentration, often used to diagnose APN, have still deficiencies in distinguishing this condition from others13). Resolution of fever during treatment and increased WBC, ESR or CRP have been regarded as useful clinical indicators in predicting the presence or severity of APN14-16), but recent studies17,18) have emphasized the importance of imaging methods to confirm the diagnosis in these children.
Main focus of the previous studies was finding out the best diagnostic methods and predictive values to enable early diagnosis and appropriate treatment of APN to diminish renal scarring. Young children are especially prone to APN8), and renal scarring as a complication of APN has been estimated to occur in as many as 64% of affected pediatric kidneys3,19). Thus, when APN is suspected in children, imaging tools to confirm the diagnosis early and effectively are still more important.
Although 99mTc-DMSA scintigraphy includes sedation and radiologic exposure, it has been giving higher diagnostic sensitivity and fewer side effects than other imaging methods, making it the best one currently available for the diagnosis of APN20,21). Renal ultrasonography has been the first imaging method used to assess children under suspicion of APN, but is deficient in detecting renal parenchyma involvement. Power Doppler ultrasonography is superior to conventional ones, but holds little likelihood of replacing 99mTc-DMSA scintigraphy6,22-24).
Recent improvements in MDCT, however, enable the calculation of the volume of a wide region using thin slices in a brief time, allowing the production of not only high-quality axial images but also high-resolution 3D images. Thus, it is necessary to reconsider the utility of MDCT for diagnosing APN in children. While, classical CT cannot provide high-resolution images of the kidney due to respiratory interference in children, MDCT has made possible such high-resolution images by scanning at a higher rate enough to overcome respiratory motion.
First generation CT had the drawback of severe respiratory interference due to long scanning times. The development of computed axial tomography accelerated advances in CT, resulting in the development of second to fifth generation machines of radically reduced scanning times. Rather than discontinuous images of old CT, the helical CT can produce images of specific region in series, vastly improving the quality.
The mechanism by which the recent MDCT can operate without respiratory interference and produce high-resolution images are as follows. In single-detector row CT (SDCT or single spiral CT), rotation of the CT gentry can obtain information on a channel. To obtain 32 slices, for example, the CT gantry should rotate 32 times in a pitch; thus, if each rotation takes one second, this would take a total of 32 seconds. In contrast, MDCT utilizes multiple rows of detectors, which obtain information of multiple images from a single rotation of the CT gantry. When an array of detectors can obtain information on 4 channels simultaneously from each rotation, the machine is called 4-channel scanner, and if it can obtain information on 16 channels it is called as 16-channel scanner. That is, SDCT involves 32 gantry rotations to obtain 32 slices, whereas 4- and 16-channel MDCT can obtain the same information from 8 and 2 rotations, respectively, thus yielding the same information in much shorter times.
The results of imaging study for children with APN were reported to vary according to the age of patients. Because traditional CT could not produce high resolution images of the kidney, the presence of APN have been made definitively by DMSA, although its detection rate was lower in younger children than in older. For example, the detection rates of APN in suspected children having this condition were found to be 55% in children aged <1 year, 79% in those aged 1 to 5 years, and 69% in those aged over 5 years, respectively25).
We have experienced pediatric patients with APN confirmed by MDCT during evaluation of their acute abdomen in emergency room, but the additional DMSA scan as a routine diagnostic work-up of APN failed to show up any renal lesions. So we tried to find out the diagnostic handicap of DMSA scan in comparing to MDCT. We retrospectively gathered 81 children of such conditions, who received both MDCT and DMSA scan, and evaluated diagnostic value of DMSA scan in detecting the MDCT-confirmed renal lesions. Biologic parameters for confirming the diagnosis of APN were not necessary condition in our study, but most patients fulfilled the inclusion criteria for the diagnosis, such as fever, pyuria, and positive urine culture, except ones who had been taking antibiotics already and showed normal urine findings.
In all 81 patients who showed APN lesions on MDCT, the additional DMSA scans were done but failed to reveal any abnormal lesion in 26 patients, false negative rate of 32.1%. Of these 26 patients, 17 (65.4%) were under age of 1 year and 19 (73.0%) were below 2 years. Consistent with previous reports, the result of our study supports that DMSA scan is less reliable than MDCT in diagnosing APN of children, particularly for aged <2 years. The laboratory and clinical findings did not differ between patients, aged <2 and ≥2 years.
A diagnostic method that gives immediate results is desirable since early diagnosis of APN is critical for saving kidneys in children. DMSA has long been preferred in children, due to its high diagnostic sensitivity compared to other imaging modalities. However, DMSA has several drawbacks, including its requirements for professional personnel and facilities for handling nuclear contrast agent; at least 4-hour-preparation time from the injection of contrast agent to its absorption; exposure to radiation; and a requirement for anesthesia. While, MDCT can be performed at any time of the day, and can be performed quickly, even in the emergency room. Furthermore, in contrast to DMSA, MDCT can show anatomical features of renal lesion and differentiate inflammation or abscess including tissues around the kidney by providing high sensitivity and high-quality images even for young children <2 years.
In another study of the authors, the recovery rates of renal defects on DMSA scan in children with APN was 66.3%26), but we cannot say that this means substantial recovery of the inflammatory kidneys, because there are sufficient possibilities of parenchyma loss invisible on DMSA scan. We don't know about the nature of the renal lesions detected only by MDCT but hidden from DMSA scan. It may be due to poor resolution power of DMSA, or from the various configurations of renal lesions; diffuse, small, scattered, or bilateral. And also we suggest that there may be a certain inflammatory phase of APN refractory to DMSA scan, presumably very early stage of inflammation. Low radiation dose protocol is still more preferred in children. With the modern MDCT technology, it is possible that low-dose CT can produce high resolution images even in young children. And 64-slice CT can yield excellent diagnostic imaging quality without an increase in radiation dose compared to the lower level multi-slice scanners27,28).
With the advantages of readiness, simplicity, high resolution, and need for brief anesthesia, MDCT would be widely adopted as the first-line diagnostic tool for APN in near future, especially for the pediatric cases presented with acute abdomen or severe complications. And this will provide a chance to prevent renal injuries caused by misdiagnosis or incomplete treatment of APN in childhood.
References
1. Talner LB, Davidson AJ, Lebowitz RL, Dalla Palma L, Goldman SM. Acute pyelonephritis: can we agree on terminology? Radiology 1994;192:297–305.


3. Jakobsson B, Berg U, Svensson L. Renal scarring after acute pyelonephritis. Arch Dis Child 1994;70:111–115.



4. Huang DT, Huang FY, Tsai TC, Tsai JD, Chiu NC, Lin CC. Clinical differentiation of acute pyelonephritis from lower urinary tract infection in children. J Microbiol Immunol Infect 2007;40:513–517.

5. Coulthard MG, Lambert HJ, Keir MJ. Do systemic symptoms predict the risk of kidney scarring after urinary tract infection? Arch Dis Child 2009;94:278–281.


6. Bykov S, Chervinsky L, Smolkin V, Halevi R, Garty I. Power Doppler sonography versus Tc-99m DMSA scintigraphy for diagnosing acute pyelonephritis in children: are these two methods comparable? Clin Nucl Med 2003;28:198–203.

7. Sattari A, Kampouridis S, Damry N, Hainaux B, Ham HR, Vandewalle JC, et al. CT and 99mTc-DMSA scintigraphy in adult acute pyelonephritis: a comparative study. J Comput Assist Tomogr 2000;24:600–604.


8. Lee SW, Baek SY, Lee SJ. CT of acute pyelonephritis in children: comparison with Tc-99m DMSA scintigraphy. J Korean Radiol Soc 1998;38:933–939.

9. Pylkkänen J, Vilska J, Koskimies O. The value of level diagnosis of childhood urinary tract infection in predicting renal injury. Acta Paediatr Scand 1981;70:879–883.


11. Jacobson SH, Eklöf O, Lins LE, Wikstad I, Winberg J. Long-term prognosis of post-infectious renal scarring in relation to radiological findings in childhood: a 27-year follow-up. Pediatr Nephrol 1992;6:19–24.


12. Wikstad I, Hannerz L, Karlsson A, Eklöf AC, Olling S, Aperia A. 99mTechnetium dimercaptosuccinic acid scintigraphy in the diagnosis of acute pyelonephritis in rats. Pediatr Nephrol 1990;4:331–334.


13. Bloomfield P, Hodson EM, Craig JC. Antibiotics for acute pyelonephritis in children. Cochrane Database Syst Rev 2005;(1): CD003772

14. Bachur R. Nonresponders: prolonged fever among infants with urinary tract infections. Pediatrics 2000;105:E59


15. Stokland E, Hellström M, Jacobsson B, Jodal U, Lundgren P, Sixt R. Early 99mTc dimercaptosuccinic acid (DMSA) scintigraphy in symptomatic first-time urinary tract infection. Acta Paediatr 1996;85:430–436.


16. Benador D, Benador N, Slosman DO, Nusslé D, Mermillod B, Girardin E. Cortical scintigraphy in the evaluation of renal parenchymal changes in children with pyelonephritis. J Pediatr 1994;124:17–20.


17. Wallin L, Helin I, Bajc M. Follow-up of acute pyelonephritis in children by Tc-99m DMSA scintigraphy: quantitative and qualitative assessment. Clin Nucl Med 2001;26:423–432.


18. Hitzel A, Liard A, Véra P, Manrique A, Menard JF, Dacher JN. Color and power Doppler sonography versus DMSA scintigraphy in acute pyelonephritis and in prediction of renal scarring. J Nucl Med 2002;43:27–32.

20. Majd M, Rushton HG. Renal cortical scintigraphy in the diagnosis of acute pyelonephritis. Semin Nucl Med 1992;22:98–111.


21. Eggli DF, Tulchinsky M. Scintigraphic evaluation of pediatric urinary tract infection. Semin Nucl Med 1993;23:199–218.


22. Biggi A, Dardanelli L, Pomero G, Cussino P, Noello C, Sernia O, et al. Acute renal cortical scintigraphy in children with a first urinary tract infection. Pediatr Nephrol 2001;16:733–738.


24. Hoberman A, Charron M, Hickey RW, Baskin M, Kearney DH, Wald ER. Imaging studies after a first febrile urinary tract infection in young children. N Engl J Med 2003;348:195–202.


25. Benador D, Benador N, Slosman D, Mermillod B, Girardin E. Are younger children at highest risk of renal sequelae after pyelonephritis? Lancet 1997;349:17–19.


26. Seon YS, Kwon DG, Shin YH, Pai KS. Prognostic factors of renal defects on the initial DMSA scan in children with acute pyelonephritis. J Korean Soc Pediatr Nephrol 2010;14:195–202.

Fig. 1
Rate of detection failure on 99mTc-dimercaptosuccinic acid (DMSA) scan for renal lesions which were confirmed by multi-detector row computed tomogram in children with of acute pyelonephritis (APN) and its differences by age groups.Dark bar represents the number of children with MDCT-proven pyelonephritis in each age group, while the white bar represents the portion of children who fail to show lesions through 99mTc-DMSA scan.

Fig. 2
Example images of multi-detector row computed tomogram (MDCT) and dimercaptosuccinic acid (DMSA) scan in a 8-month-old boy with focal pyelonephritis. Coronal reformation image of MDCT is showing the lesion with decreased contrast enhancement in the upper portion of left kidney (A). 99mTc-DMSA scintigraphy resulted in normal finding on left kidney, failing to show the inflammatory lesion visible on MDCT (B). Ant. anterior; Post, posterior; Rpo, right opsterior; Lpo, left posterior.
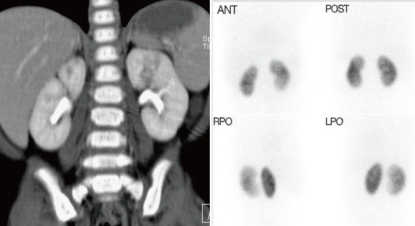
Table 2
Clinical and Biochemical Data of 26 Patients with MDCT-Confirmed Acute Pyelonephritis Who Showed Normal Findings on DMSA Scan
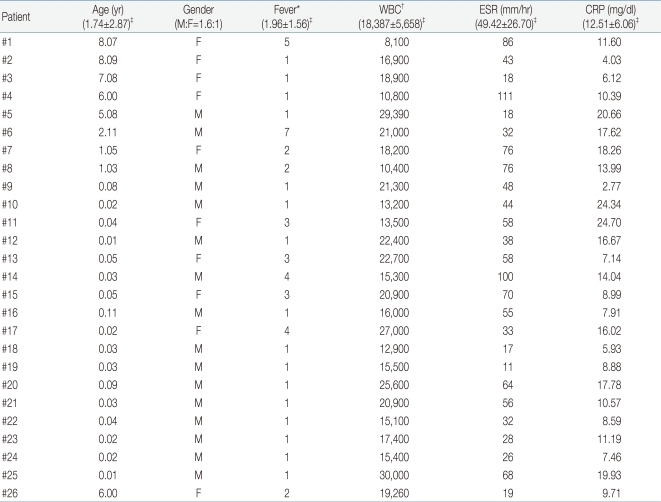
Table 3
Comparison of Clinical and Biochemical Parameters of Patients with MDCT-Confirmed Acute Pyelonephritis According to the Positivity of DMSA Results
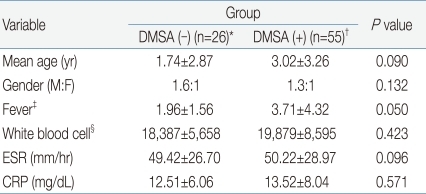
Values are presented as mean±SD.
Gender predilection was analyzed by the chi-square test.
MDCT, multi-detector row computed tomogram; DMSA, 99mTc-dimercaptosuccinic acid scan, ESR, erythrocyte sedimentation rate; CRP, C-reactive protein.
*Consisted of patients with acute pyelonephritis who showed positive image findings on MDCT and DMSA. †Consisted of patients positive on MDCT but not on DMSA. ‡Fever, febrile days before admission. §Leukocyte counts on peripheral blood examination.




 PDF Links
PDF Links PubReader
PubReader PubMed
PubMed Download Citation
Download Citation


