Article Contents
| Korean J Pediatr > Volume 54(5); 2011 |
Abstract
Purpose
This study was conducted to investigate the immune responses of children with moderate and severe novel influenza A virus (H1N1) pneumonia, and to compare their clinical and immunological findings with those of control subjects.
Methods
Thirty-two admitted patients with H1N1 pneumonia were enrolled in the study. The clinical profiles, humoral and cell-mediated immune responses of the 16 H1N1 pneumonia patients who were admitted to the pediatric intensive care unit (severe pneumonia group), 16 H1N1 pneumonia patients admitted to the pediatric general ward (moderate pneumonia group) and 13 control subjects (control group) were measured.
Results
Total lymphocyte counts were significantly lower in patients with H1N1 pneumonia than in the control group (P=0.02). The number of CD4+ T lymphocytes was significantly lower in the severe pneumonia group (411.5±253.5/µL) than in the moderate pneumonia (644.9±291.1/µL, P=0.04) and control (902.5±461.2/µL, P=0.01) groups. However, the number of CD8+ T lymphocytes was significantly higher in the severe pneumonia group (684.2±420.8/µL) than in the moderate pneumonia (319.7±176.6/µL, P=0.02) and control (407.2±309.3/µL, P=0.03) groups. The CD4+/CD8+ T lymphocytes ratio was significantly lower in the severe pneumonia group (0.86±0.24) than in the moderate pneumonia (1.57±0.41, P=0.01) and control (1.61±0.49, P=0.01) groups. The serum levels of IgG, IgM and IgE were significantly higher in the severe pneumonia group than in the 2 other groups.
In March 2009, a novel influenza A virus (H1N1) emerged and spread worldwide to cause the first pandemic of the 21st century as declared by the World Health Organization on June 11, 20091).
Although most subjects with H1N1 infection present flu-like symptoms with a benign clinical course, some patients may have a serious respiratory illness, including pneumonia as well as respiratory distress, which may require mechanical ventilation2-4). Previous studies have reported that more than 30% of H1N1 infected children were admitted to the pediatric intensive care unit (PICU) due to severe pneumonia2, 5). However, immune responses associated with severe H1N1 pneumonia remain to be determined. Such knowledge of immune responses associated with severe H1N1 pneumonia may help clinicians identify patients and provide appropriate management.
There are few reports on the immune responses of patients with H1N1 infection6, 7). T lymphocyte function is critical to the clearance of influenza virus infection. Activation of CD8+ T lymphocytes begins to appear 3 days after infection and peaks on days 6 to 9 during the course of influenza infection8). Previous studies have revealed that CD8+T lymphocytes play a crucial role in H1N1 infection as well as seasonal influenza virus infection9-11). CD8+ T cells are important in the antiviral response to influenza via direct lysis or by the induction of inflammatory cytokines but they can show altered immune response to severe infections8). However, immune responses leading to increased severity in previously healthy children during the course of H1N1 pneumonia are not well understood.
This study was conducted to investigate the clinical and immunological manifestations of children with moderate and severe H1N1 pneumonia and to compare their clinical and immunologic findings with control subjects.
A total of 68 patients with H1N1 infection that were admitted to Korea University Anam Hospital between October 2009 and March 2010 were recruited for this study. Among them, 16 patients were admitted to the PICU because of severe pneumonia and respiratory distress (severe pneumonia group). Subjects admitted to the PICU were described as having dyspnea and hypoxemia (oxygen saturation <93% in room air) or respiratory failure requiring mechanical ventilation. Among the 52 remaining patients with H1N1 infection those who were admitted to the general ward, patients with croup, bronchitis or neurologic diseases were excluded. Children who did not agree with the participation of the clinical trial were also excluded. Finally, 16 patients with H1N1 pneumonia that were admitted to the general ward were designated as having moderate pneumonia. Thirteen children that were admitted to the Department of Pediatrics for non-infectious diseases served as control subjects; some had a past history of recurrent infection but no identifiable airway infection for 4 weeks prior to this study. Nasopharyngeal real-time reverse-transcriptase polymerase-chain-reaction (RT-PCR) was negative for respiratory viruses in all control subjects.
All parents of the patients and of the control subjects provided written informed consent for their children to participate in this study. The study protocol was approved by the Institutional Review Board of our hospital.
A complete history, a physical examination, and routine blood tests were performed on each subject at the time of study entry. H1N1 infection was confirmed by nasopharyngeal swab specimens using RT-PCR assay at the Department of Clinical Laboratory in our hospital according to the manufacturer's instructions. In addition, nasopharyngeal aspirates were tested to identify respiratory syncytial virus, adenovirus and parainfluenza virus by cultures. The diagnosis of pneumonia was based on clinical (fever, cough, dyspnea and crackles) and radiological (chest infiltrates) criteria12). Children with immune disorder, neurological disease, chronic lung disease, and co-infection by either viruses or bacteria were excluded from the study.
Peripheral blood T lymphocyte subsets were determined with a BD TriTEST kit (BD Immunocytometry Systems, San Jose, CA, USA). CD4 fluorescein isothiocyanate, CD8 phecoerythin, and CD3 peridinin chlorophyll protein were used for immunofluorescence reagents. Optimal conditions for monoclonal-antibody concentrations and incubation periods were established according to the manufacturer's instructions. Fluorocytometry was performed with a FACSCalibur (BD Immunocytometry Systems) equipped with a 488-nm blue laser and a 635-nm red diode laser for multicolor fluorescence, in addition to forward-scatter and side-scatter measurements. All fluorocytometric data were subsequently analyzed and displayed graphically using CellQuest Pro software (BD Immunocytometry Systems). The serum levels of IgG, IgM, and IgA were measured by immunoturbidimetry (Turbiquant IgG, IgM, and IgA; Behringwerke AG, Marburg, Germany). Serum total IgE levels were measured using a Coat-A-Count Total IgE IRMA (Diagnostic Products Co., Los Angeles, CA, USA) according to the manufacturer's instructions.
Data are presented as mean±SD. Becuase most of the data were not normally distributed, non-parametric analysis was used. The data were screened for differences in humoral immune responses and T lymphocyte profiles in the 3 study groups using the Kruskal-Wallis test. When significant differences were identified, individual groups were compared using the Mann-Whitney U test. All statistical analyses were performed using the SPSS statistical package (SPSS Inc., Chicago, IL, USA). A P value of less than 0.05 was considered statistically significant.
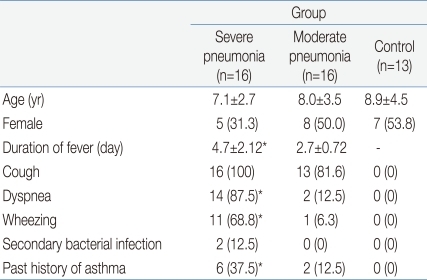
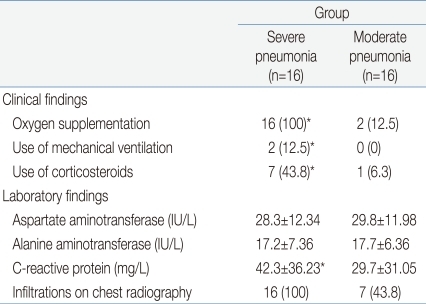
The clinical characteristics of the children in the 3 groups are summarized in Tables 1 and 2. There were no significant differences in age and sex ratio between the 3 groups. The duration of fever was significantly longer, and the use of mechanical ventilation and a past history of asthma were more common in the severe pneumonia group than in the moderate pneumonia and control groups.
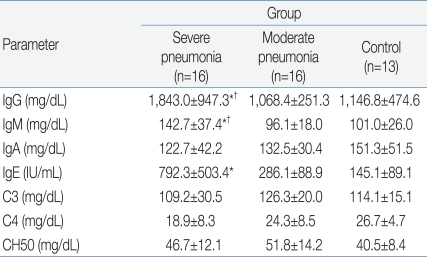
Table 3 shows the humoral immune responses of the children in the 3 groups. The mean(±SD) serum IgG level was significantly higher in the severe pneumonia group (1,843.0±947.3 mg/dL) than in the moderate pneumonia (1,068.4±251.3 mg/dL, P=0.03) and control (1,146.8±474.6 mg/dL, P=0.04) groups. The mean(±SD) serum IgM level was also significantly higher in the severe pneumonia group (142.7±37.4 mg/dL) than in the moderate pneumonia (96.1±18.0 mg/dL, P=0.04) and control (101.0±26.0 mg/dL, P=0.04) groups. The severe pneumonia group showed a significantly higher serum total IgE level (792.3±503.4 IU/mL) than the moderate pneumonia (286.1±88.9 IU/mL) and control groups (145.1±89.1 IU/mL, P=0.02). There were no significant differences in serum levels of IgA, C3, C4 and CH50 between the 3 groups.
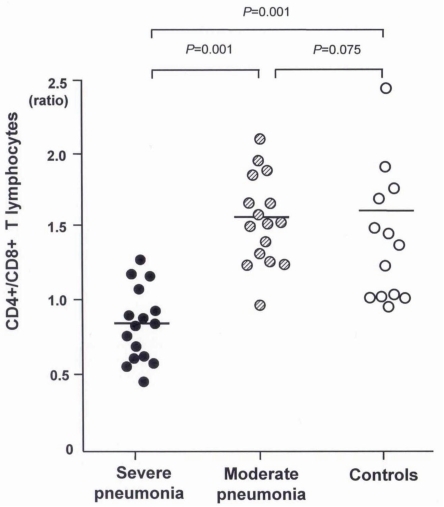
The mean(±SD) total lymphocyte counts in peripheral blood were significantly lower in the severe pneumonia (1,567.6±926.2/µL) and moderate pneumonia (1,425.6±821.4/µL) groups than in the control group (2,529.4±1,368.7/µL, P=0.03 and P=0.01, respectively). The lymphocyte subsets of the 3 groups are shown in Table 4. Children in the severe pneumonia group had a significantly lower number of CD4+ T lymphocytes (411.5±253.5/µL) than those in the children with moderate pneumonia (644.9±291.1/µL, P=0.02) and control (902.5±461.2/µL, P=0.01) groups. The number of CD8+ T lymphocytes was significantly higher in the severe pneumonia group (684.2±420.8/µL) than in the moderate pneumonia (319.7±176.6/µL, P=0.02) and control (407.2±309.3/µL, P=0.03) groups. The ratio of CD4+/CD8+ T lymphocytes was significantly decreased in the severe pneumonia group (0.86±0.24) than in the moderate pneumonia (1.57±0.41, P=0.01) and control (1.61±0.49, P=0.01) groups (Fig. 1).
The results of this study showed that patients with severe H1N1 pneumonia had prolonged fever, respiratory difficulties, decreased CD4+ T lymphocytes, increased CD8+ T lymphocytes and caused an imbalance of the CD4+/CD8+ T lymphocytes ratio during the course of infection. This is the first study to investigate the humoral and cellular immune responses of children with H1N1 pneumonia. Thirty-two children with confirmed H1N1 pneumonia were enrolled in this study, including 16 patients admitted to the PICU. The patients with severe pneumonia had dyspnea, hypoxemia or respiratory difficulties; however, there were no deaths.
In this study, patients with H1N1 pneumonia showed decreased lymphocyte counts compared to the controls, which is consistent with the results of previous studies4, 6). This finding suggests that the H1N1 virus is capable of inhibiting cellular immune function and is probably related to the induction of excessive apoptosis of lymphocytes13). Cellular immune responses to influenza, such as viral clearance8) and lymphopenia, are related to poor prognoses in patients with viral infections6, 14, 15).
Many studies have demonstrated a major role for CD8+ T lymphocytes in the control of viral infections7, 10, 16). CD8+ T lymphocytes have either beneficial or harmful effects on the virus infected host. CD8+ T lymphocyte function is critical for the resolution of respiratory viral infections; however, it has been demonstrated that T lymphocyte responses to the infected respiratory epithelium may result in significant tissue damage10). Subbarao et al.8) have reported that the activation of CD8+ T lymphocytes begins to appear 3 days after infection and peaks on days 6 to 9 during the course of influenza infection. The increase of the CD8+ T lymphocyte response to H1N1 might also lead to severe infections as shown in influenza virus infections.
Since replication of influenza viruses is restricted to the epithelial cells of the respiratory tract and systemic exposure of the immune system to influenza viruses is consequently limited, the contribution of CD8+ T lymphocytes to primary antiviral responses is not clear17). However, many studies have demonstrated a crucial role of CD8+ T lymphocytes in the clearance of infected viruses. In our study, it was found that the number of CD8+ T lymphocytes was higher in the severe pneumonia group than in the moderate pneumonia or control groups.
The CD4+/CD8+ T lymphocyte ratio was significantly decreased in the severe pneumonia group compared to the moderate pneumonia and control groups. Furthermore, the ratio of lymphocyte subpopulations in the moderate pneumonia group was within the normal range of children of the same age group reported by Robinson et al18). However, it was significantly decreased in the severe pneumonia group. These results suggest that altered immune responses with increased CD8+ T lymphocyte production or imbalance of the CD4+ and CD8+ T lymphocytes might be related to severe clinical inflammatory responses to H1N1 infection. It is unclear whether this event is temporary or permanent because the number of lymphocytes was not measured after recovery from H1N1 infection. However, this is the first demonstration of systemically altered CD4+/CD8+ T lymphocyte responses in children with severe H1N1 pneumonia.
In this study, the serum levels of IgG and IgM were higher in the severe pneumonia group than in the moderate pneumonia and control groups. Although the increased IgM titer is suggestive of a recent infection, it is not certain whether it was H1N1-specific antibody.
Another point to discuss is the relationship between asthma and the development of severe H1N1 infection19). In the subjects of this study, although there were no patients with current asthma, a past history of asthma was more common in the severe H1N1 pneumonia group (n=6, 37.5%) than in the moderate H1N1 pneumonia group (n=2, 12.5%, P=0.01). There were no patients with a past history of asthma among the control subjects.
One of the limitations of this study is the time point at which measurements were made. The number of CD+ T lymphocytes may differ according to the amount of time elapsed since H1N1 infection8). In this study, lymphocyte counts were performed on admission, and we could not monitor the counts until they recovered to the normal range. In addition, some subjects in the control group had a history of recurrent infections; however, none of them were within the 4 weeks to the study.
The inappropriate CD4+/CD8+ T lymphocyte responses and humoral immunity are likely to intensify the pathological process10). Evaluation of immune responses including CD4+ and CD8+ T lymphocytes in patients with H1N1 infection will provide information that can aid in the development of appropriate strategies for the management of patients with this infection.
References
1. Chan M. World now at the start of 2009 influenza pandemic [Internet]. c2011;cited 2011 Mon Date. Geneva: World Health Organization, Available from: http://www.who.int/mediacentre/news/statements/2009/h1n1_pandemic_phase6_20090611/en/index.html.
2. Miroballi Y, Baird JS, Zackai S, Cannon JM, Messina M, Ravindranath T, et al. Novel influenza A(H1N1) in a pediatric health care facility in New York City during the first wave of the 2009 pandemic. Arch Pediatr Adolesc Med 2010;164:24–30.

3. Novel Swine-Origin Influenza A (H1N1) Virus Investigation Team. Dawood FS, Jain S, Finelli L, Shaw MW, Lindstrom S, et al. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med 2009;360:2605–2615.


4. Perez-Padilla R, de la Rosa-Zamboni D, Ponce de Leon S, Hernandez M, Quiñones-Falconi F, Bautista E, et al. Pneumonia and respiratory failure from swine-origin influenza A (H1N1) in Mexico. N Engl J Med 2009;361:680–689.


5. Louie JK, Acosta M, Winter K, Jean C, Gavali S, Schechter R, et al. Factors associated with death or hospitalization due to pandemic 2009 influenza A (H1N1) infection in California. JAMA 2009;302:1896–1902.


6. Cui W, Zhao H, Lu X, Wen Y, Zhou Y, Deng B, et al. Factors associated with death in hospitalized pneumonia patients with 2009 H1N1 influenza in Shenyang, China. BMC Infect Dis 2010;10:145



7. Giamarellos-Bourboulis EJ, Raftogiannis M, Antonopoulou A, Baziaka F, Koutoukas P, Savva A, et al. Effect of the novel influenza A (H1N1) virus in the human immune system. PLoS One 2009;4:e8393



8. Subbarao K, Murphy BR, Fauci AS. Development of effective vaccines against pandemic influenza. Immunity 2006;24:5–9.


9. Doherty PC. Cytotoxic T cell effector and memory function in viral immunity. Curr Top Microbiol Immunol 1996;206:1–14.

10. Mauad T, Hajjar LA, Callegari GD, da Silva LF, Schout D, Galas FR, et al. Lung pathology in fatal novel human influenza A (H1N1) infection. Am J Respir Crit Care Med 2010;181:72–79.


11. Bruder D, Srikiatkhachorn A, Enelow RI. Cellular immunity and lung injury in respiratory virus infection. Viral Immunol 2006;19:147–155.


12. Jadavji T, Law B, Lebel MH, Kennedy WA, Gold R, Wang EE. A practical guide for the diagnosis and treatment of pediatric pneumonia. CMAJ 1997;156:S703–S711.

13. Nichols JE, Niles JA, Roberts NJ Jr. Human lymphocyte apoptosis after exposure to influenza A virus. J Virol 2001;75:5921–5929.



14. Tran TH, Nguyen TL, Nguyen TD, Luong TS, Pham PM, Nguyen VC, et al. Avian influenza A (H5N1) in 10 patients in Vietnam. N Engl J Med 2004;350:1179–1188.


15. Chotpitayasunondh T, Ungchusak K, Hanshaoworakul W, Chunsuthiwat S, Sawanpanyalert P, Kijphati R, et al. Human disease from influenza A (H5N1), Thailand, 2004. Emerg Infect Dis 2005;11:201–209.



16. Tu W, Mao H, Zheng J, Liu Y, Chiu SS, Qin G, et al. Cytotoxic T lymphocytes established by seasonal human influenza cross-react against 2009 pandemic H1N1 influenza virus. J Virol 2010;84:6527–6535.



17. Moskophidis D, Kioussis D. Contribution of virus-specific CD8+ cytotoxic T cells to virus clearance or pathologic manifestations of influenza virus infection in a T cell receptor transgenic mouse model. J Exp Med 1998;188:223–232.



Fig. 1
The CD4+/CD8+ T lymphocyte ratio is lower in children with the severe pneumonia than those of the moderate pneumonia and control groups.




 PDF Links
PDF Links PubReader
PubReader PubMed
PubMed Download Citation
Download Citation


