Introduction
While most of the children who presented with primary nephrotic syndrome (NS) respond to steroid treatment, 10 to 20% of these children do not achieve remission despite receiving second or third-line of treatment (Fig. 1), and more than half of the patients progress to end-stage renal disease (ESRD) within 10 years1). Thus, various modalities have been tried to induce remission in these patients. In this review, several commonly-practiced treatments will be reviewed while simultaneously following the progression of a clinical case of pediatric steroid-resistant nephrotic syndrome (SRNS).
Pharmacological treatment of SRNS
1. Case; Part 1
A 5-years-old boy was transferred to our center for treatment of steroid-resistant nephrotic syndrome. He had developed edema with proteinuria and did not respond to oral steroid treatment. Kidney biopsy revealed focal segmental glomerulosclerosis with segmental mesangial hypercellularity and segmental sclerosis in 7% of glomeruli. To achieve remission, intravenous methylprednisolone pulse therapy and cyclosporine treatment were attempted (Fig. 2).
1) SRNS
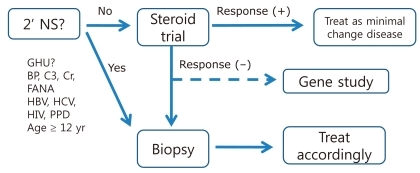
SRNS is defined as NS that does not respond to one month's treatment with oral prednisolone at a dosage of 60 mg/m2/day. When SRNS is suspected, a meticulous search for the possibility of concurrent infection (e.g., sinusitis and skin infection), drug interaction (e.g., antiepileptic drugs), inappropriate dosage, or compliance problem is necessary. If those possibilities are ruled out, tissue diagnosis from a kidney biopsy is the next step. Histological findings of SRNS are, and occasionally, a secondary glomerulopathy such as amyloidosis is unexpectedly found. At the same time, mutational analysis for genes known to cause SRNS is recommended. Mutations of WT1, INF2, LAMB2, ACTN4, NPHS1, and NPHS2 have been found in Korean children with SRNS by Cheong et al.2-5). While more aggressive treatment is required to achieve remission in the case of SRNS of unknown cause (primary SRNS) to achieve remission, such an aggressive treatment is not effective for those with SRNSthat arises from genetic causes; therefore genetic testing may shield these children from the unnecessary side effects of immunosuppressive medications (Fig. 3).
2) Methylprednisolone pulse treatment
When oral prednisolone treatment fails, intravenous methylprednisolone pulse therapy (30 mg/kg, every other day, 6 doses in total) is commonly tried. The original treatment protocol developed by Mendoza et al.6); however, remission rates as high as 70% were reported with this protocol. The current practice involves the administration of 3 to 6 doses of high-dose intravenous methylprednisolone before kidney biopsy, and patients who respond to this treatment are often regarded as responsive to steroid therapy. Commonly encountered side effects of methylprednisolone pulse treatment are infection, Cushing's syndrome, hypertension, glucose intolerance, and arrhythmia during infusion.
3) Calcineurin inhibitors (CNI)
Cyclosporine and tacrolimus (FK-506) were originally introduced as immunosuppressive agents for allograft transplantation due to their inhibitory effect on calcineurin, a key signal transduction molecule activating T lymphocytes. In the past, the anti-proteinuric effect of calcineurin inhibitors (CNIs) was believed to arise from their immunosuppressive effect on lymphocytes7). However, CNI CNIs have recently been found to stabilize the cytoskeleton of glomerular epithelial cells (podocytes) and thereby reduce glomerular proteinuria8). This effect explains why cyclosporine has partial success in some cases of proteinuria of proteinuria arising from genetic causes9). The response rate of SRNS to cyclosporine is roughly 40 to 60. A typical SRNS treatment protocol using cyclosporine involves the administration of cyclosporine (150 to 200 mg/m2/day) and prednisolone (30 mg/m2/day) for 1 month, followed by alternate-day prednisolone for 5 months; this has been shown to result in complete remission in 42% of recipients within the first 6 months10). Cyclosporine has a well-known spectrum of side effects such as nephrotoxicity, infection, hypertension, hyperkalemia, renal tubular acidosis, tremor, glucose intolerance, gum hypertrophy, and hirsutism. The therapeutic drug level (trough) of cyclosporine is <100-200 ng/mL.
Another CNI, tacrolimus, is also used in the treatment of SRNS, although Korean Food and Drug Administration has not approved this medication for treatment of NS11). The dosage of tacrolimus for SRNS is 0.05 to 1 mg/kg/day with a trough level <5 to 10 µg/L. Tacrolimus has a similar spectrum of side effects as cyclosporine but does not cause gum hypertrophy or hirsutism.
4) Alkylating agents and anti-proliferative agents
While cyclophosphamide or chlorambucyl have been used in early reports; however, a recent review by the Children's Nephrotic Syndrome Consensus Conference concluded that these alkylating agents were not superior to steroid mono-therapy12). Additionally, mofetil13) and sirolimus14) have also been tried recently with moderate results.
Non-conventional treatment of SRNS
1. Case; Part 2 (Fig. 4)
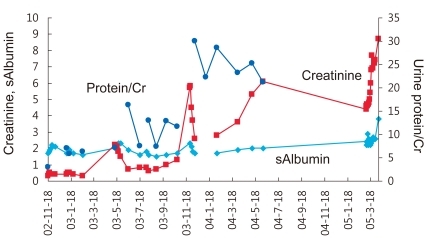
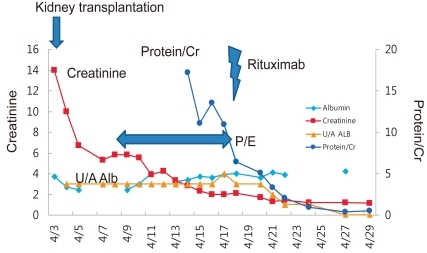
Despite various treatments, the patient's proteinuria and hypoalbuminemia did not disappear and instead progressed to endstage renal disease (ESRD) in 2 years and 1 month (Fig. 1). Peritoneal dialysis was started at the age of 8 years and 3 months. After 4 years, the patient received cadaveric donor kidney transplantation. Following the surgery, his serum creatinine level began to drop, but soon increased again to staggering levels. At the same time, his serum albumin level began to decrease as well. Urine albumin levels were found to be 3+.
1) Renal replacement therapy for children with nephrotic syndrome
(1) Dialysis
When kidney function deteriorates and progresses to ESRD, there are 3 options for renal replacement: hemodialysis, peritoneal dialysis, and transplantation. Peritoneal dialysis requires less strict diet control and enables a more flexible life style; therefore, peritoneal dialysis is preferred to hemodialysis in pediatric patients, despite the risk of complicating peritonitis. Protein loss through the kidneys in children with SRNS diminishes with the deterioration of kidney function and their intractable edema improves accordingly. On peritoneal dialysis. However, protein loss through the peritoneal membrane may develop with peritoneal dialysis, especially in children with SRNS, as documented at our center15). Therefore, the possibility of protein malnutrition should be considered and adequate nutritional support is required.
(2) Kidney transplantation
Kidney transplantation is by far the most advantageous modality of renal replacement for children, since only with kidney transplantation normal or near-normal growth can be achieved only with kidney transplantation. Currently, the outcomes of kidney transplantation are excellent with one-year survival of 93%, 10-year survival rates of around ~80%, and a median graft survival time of >15 years. However, children with SRNSrun the risk of recurrence of NS following transplantation, as illustrated in the case. Recurrence often precipitates delayed graft function, acute rejection, and poor graft outcomes16). Nevertheless, the expected graft survival rate of kidney allograft in patients with SRNS is as good as that of cadaveric donors; therefore, SRNS is not a contraindication for kidney transplantation.
(3) Recurrence of SRNS after transplantation
Risk factors for SRNS recurrence following transplantation include childhood-onset SRNS, age of onset greater than 5-6 years, rapid progression to ESRD, and recurrence following a previous allograft17). On the other hand, cases of secondary SRNS, such as those secondary to infection, medication, reflux nephropathy, or obesity, and SRNS arising from genetic causes are not likely to recur18).
Recurrence often develops within 48 hours of transplantation. Other possible diagnoses of post-transplant proteinuria include chronic allograft rejection, de novo glomerulopathy, and proteinuria from the native kidneys. Nevertheless, significant proteinuria following kidney transplantation in SRNS patients should be presumed to be a recurrence. In this case, aggressive and timely treatment with plasmapheresis and/or high-dose cyclosporine (250 to 350 ng/mL) or rituximab is required, since treatment within 48 hours of recurrence is the only modifiable factor that can effect the remission of recurrent SRNS. For prompt detection of recurrence, meticulous monitoring of pediatric SRNS patients is required following kidney transplantation; urinalysis with protein quantitation must be performed everyday for 2 weeks after transplantation, followed by testing once a week for 1 month, then once a month for 1 year17).
2) Plasmapheresis
Observations of SRNS recurrence following graft resulted in the introduction of the concept of a 'circulating permeability factor' as a pathogenic mechanism of SRNS19). Thus, the first-line treatment to avoid recurrence is to remove the 'permeability factor' by plasmapheresis therapy. Plasmapheresis replaces patient's plasma containing the 'permeability factor' with 4 to 5% albumin solution or fresh frozen plasma. Immediate initiation of this treatment may induce remission in 50 to 90% of recurrent cases20).
Reports of favorable outcomes of plasmapheresis in patients with recurrent SRNS resulted in the application of this treatment modality in primary SRNS of native kidneys, with success in some cases21). However, it is not yet possible to predict patient responsiveness to this procedure, and the effectiveness of pre-emptive plasmapheresis to prevent SRNS recurrence following transplantation has not yet been established.
3) Rituximab
Rituximab is a monoclonal antibody against CD20, a marker of B cells, which is manufactured as a therapeutic agent for B cell lymphoma. Following the report of a case of post-transplant lymphoproliferative disease where rituximab treatment eradicated both recurrent SRNS and B cell lymphoma after kidney transplantation22), this biological agent has been used for SRNS as a rescue treatment23) with success rate of 20 to 50%. However, treatment indications and an optimal rituximab treatment protocol have not yet been established, and significant adverse effects such as opportunistic infections and rituximab-associated lung injury24) have been reported.
Conclusion
Case; Part 3
The patient was treated with plasmapheresis and his proteinuria and hypoalbuminemia improved gradually. To eradicate his nephrotic syndrome, we gave him one dose of rituximab, and complete remission was achieved. He has been in a state of remission for more than two years.
Although the prognosis of SRNS is complicated and unfavorable, intensive treatment in the early stage of the disease may achieve remission in more than half of the patients. Thus, timely referral of these patients to pediatric nephrology specialists for histological and genetic diagnosis and treatment is highly recommended.




 PDF Links
PDF Links PubReader
PubReader PubMed
PubMed Download Citation
Download Citation


