Article Contents
| Korean J Pediatr > Volume 54(10); 2011 |
Abstract
Purpose
Few studies have been conducted on the recent status of infectious mononucleosis (IM) in Korean children. The aim of this study was to evaluate the recent trend in the clinical manifestations of Epstein-Barr virus (EBV)-associated IM as well as the clinical differences according to age.
Methods
A retrospective study was performed on 81 children hospitalized with EBV-associated IM who fulfilled the serological criteria for the diagnosis of EBV infection (viral capsid antigen immunoglobulin M positive). The patients were divided into 3 age groups: <5 years, 5 to 9 years, and ≥10 years. We evaluated the recent trend in clinical manifestations and the differences in clinical and laboratory findings among the 3 age groups.
Results
Thirty (37%) children were under 5 years of age, 38 (46.9%) were 5 to 9 years of age, and 13 (16%) were 10 years of age or older. The differences in the symptoms and signs among the 3 age groups were not statistically significant, except for headache. The mean duration of fever was 7.7 days (range, 0 to 18 days). A comparison of liver enzyme elevation among the age groups showed an association with advancing age (26.6%, 63.1%, and 76.9%, respectively, P=0.04)
Conclusion
This study showed that EBV-associated IM in Korean children continues to occur mostly in children under 10 years of age. In children with EBV-associated IM, the incidence of headache and liver enzyme elevation, the duration of fever, and the proportion of females to males were all positively associated with advancing age.
Infectious mononucleosis is best known as a clinical syndrome caused by Epstein-Barr virus (EBV). It is characterized by systemic somatic complaints consisting primarily of fatigue, malaise, fever, sore throat and generalized lymphadenopathy. EBV causes more than 90% of cases of infectious mononucleosis (IM). Infection with EBV in developing countries and among socioeconomically disadvantaged populations of developed countries usually occurs during infancy and early childhood1), and 80 to 100% of children are seropositive by 3 to 6 years of age2,3). In economically privileged communities and developed countries, primary infection occurs later in life, often between the ages of 10 and 30. Those cases are more often associated with clinical symptoms such as fever, sore throat, lymphadenopathy, malaise, and headache3,4).
In Korea, the age-specific seroprevalence of antibodies to EBV was found to change over time. A seroepidemiologic study in 1977 on 137 Korean children revealed that the seroprevalence of antibodies to EBV rose rapidly between 1 and 5 years, reaching 100% by 5 years5). In 1994, the seropositivity was 84.5% in 5 and 6-year-old children and 100% in children more than 10 years of age6). There are only several clinical studies about EBV-associated IM in Korea that were done about 15 years ago7-9). Few studies about the recent status of infectious mononucleosis (IM) in Korean children have been conducted.
The aim of this study was to evaluate the recent trend of clinical manifestations and differences in the clinical and laboratory findings of EBV-associated IM according to the age of children.
We retrospectively collected cases on hospitalized patients younger than 18 years old with characteristic symptoms of IM and serologically diagnosed EBV-associated IM at Soonchunhyang University Hospital in Bucheon during a 9-year period from 2001 to 2009. All patients satisfied the diagnostic criteria of EBV-associated IM as follows: 1) presence of as least three of the following clinical manifestations: fever, tonsillopharyngitis, sore throat, cervical lymphadenopathy, hepatomegaly or splenomegaly; 2) serologic profile of primary EBV infection: present of immunoglobulin (Ig) M to EBV viral capsid antigen (VCA). Clinical and laboratory data were collected retrospectively and compared to previous studies to evaluate the differences between the clinical and laboratory findings of patients of different ages. We divided patients into three age groups: <5 years, 5 to 9 years and ≥10 years.
Primary EBV infection was diagnosed if the patient was anti-VCA IgM positive. Anti-VCA IgM was detected by ImmunoDOT Immunoassy test system (GenBio, San Diego, CA, USA). Some patients were checked for anti-VCA IgG, early antigen, Epstein-Barr nuclear antigen, and peripheral blood morphology was reviewed.
Statistical analysis was performed by use of the SPSS ver. 11.0 (SPSS Inc., Chicago, IL, USA). Chi square test and Fisher's exact test were used to examine differences in clinical characteristics among the different age groups. Age group differences in serum liver enzymes were tested by Kruskal-Wallis test and Dunnett's post test. A P value of less than 0.05 was considered statistically significant.
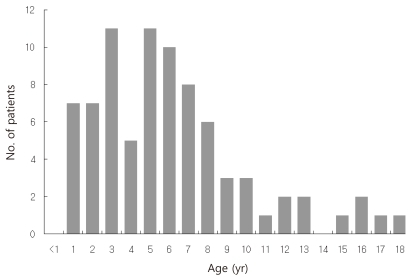
Age ranged from 1 to 18 years with a mean of 6.17 years, with the peak incidence at 3 years and 5 years of age (Fig. 1). EBV-associated IM markedly decreased after 9 years of age. Thirty (37%) children were younger than 5 years, 38 (46.9%) were 5 to 9 years, and 13 (16%) were equal to or older than 10 years of age. There were 49 male and 32 female, with a overall male-to-female ratio of 1.53:1. The proportion of female patients increased with advancing age. The male to female ratio was 2.75:1 in children younger than 5 years and 0.63 in children older than 10 years of age (Table 1).
The monthly distribution of EBV-associated IM was also analyzed and revealed peak incidences in February and August. It also frequently occurred in April, May, September and October without any specific seasonal variation.
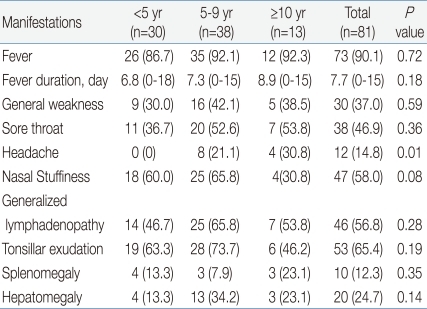
Seventy-three (90.1%) of 81 children had fever. The mean duration of fever was 7.7 days (range, 0 to 18 days), and 14.8% of patients suffered from fever longer than 10 days. Mean fever duration by age group was 6.8 days in <5 years, 7.3 days in 5 to 9 years, and 8.9 days in ≥10 years of age, showing a longer duration of fever in older children.
Sore throat and generalized lymphadenopathy were detected in 38 (46.9%) and 46 (56.8%) children, with no difference between the three age groups. Splenomegaly and hepatomegaly were present in 10 (12.3%) and 20 (24.7%) children, with similar occurrence among the three age groups. Headache was found in 0% of <5 years, 21.1% of 5 to 9 years and 30.8% of ≥10 years of age. The incidence of headache was higher with advancing age with statistical significance.
All patients recovered with conservative treatment. No children had significant complications such as airway obstruction, splenic hemorrhage or rupture, or neurologic complications (Table 2).
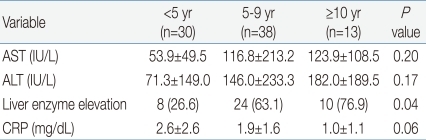
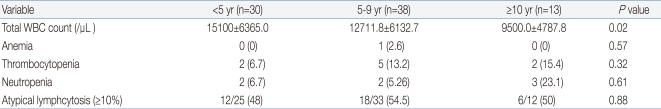
White blood cell (WBC) ranged from 2,100 to 31,200/mm3 (mean, 13,080/mm3), with leukocytosis (WBC ≥10,000/mm3) in 69.1% of the patients. The younger age group had significantly higher WBC counts than the older age group. The younger age group had higher C-reactive protein levels, although the difference was not statistically significant. There was no significant difference in the levels of alanine aminotransferase (ALT) or aspartate aminotransferase (AST) among the three age groups. However, the occurrence of elevated serum liver enzymes, defined as ALT ≥50 IU/L and/or AST ≥50 IU/L, was observed more frequently in the older age group (P=0.04): 8 (26.6%) <5 years, 24 (63.1%) 5 to 9 years and 10 (76.9%) ≥10 years. About 50% of the patients had atypical lymphocytosis (above 10% of total WBC count), with no difference among the three age groups. Mild thrombocytopenia (platelet <150,000/µL) without bleeding complications was present in 8 (9.9%) patients, with no difference among the three age groups. Only one patient had significant anemia (hemoglobin 6.9 g/dL). Neutropenia (absolute neutrophil count <1,500/µL) was present in 7 (8.6%) patients. There was no patient with pancytopenia (Tables 3, 4).
We studied the clinical and laboratory presentation of 81 patients with EBV VCA IgM positive IM patients ages 13 months to 18 years. In our study of 81 Korean children, EBV-associated IM presented at all ages, from infants to adolescents. The peak incidence occurred at 3 to 6 years, followed by 1 to 2 years and 7 to 8 years of age. That result was similar to previous clinical studies of IM in Korean children that revealed the peak incidence to be 3 to 8 years of age7-9). In Chinese and Taiwanese studies of childhood EBV-associated IM, most cases presented at 2 to 5 years of age, showing that IM in Korea presents in children a little older than in those in China and Taiwan10,11). Unlike the developed countries where EBV-associated IM predominantly occurs in adolescents and young adults because of delayed exposure to EBV, this study showed that IM still occurs mostly in young children less than 10 years of age in Korean children.
The sex ratio of EBV-associated IM among child was known as approximately equal8). But in this study, the boys younger than 5 years old more frequently developed EBV-associated IM than the girls (male-to-female ratio, 2.75:1). The proportion of female patients increased with advancing age. The gender distribution was similar to that of the Chinese children with EBV-associated IM10). The cause of the difference in gender distribution is unclear.
In addition, the relative incidence of IM closely correlates with the frequency of EBV seroconversion at different ages. The earliest EBV-associated IM case occurred in an infant aged 13 months in our study. The low rate of seroconversion in infants younger than 1 year is thought to be related to the protection of the infants by maternal antibodies, which was first demonstrated by prospective studies of primary EBV infections in African, American and Chinese infants12-14).
We could not find a definitive seasonal occurrence; peak incidences occurred in February and August. There are only a few clinical studies of IM in Korea. Only one Korean study showed a peak incidence during the autumn months8).
We compared clinical findings between three age groups, and some differences were noted. In older children equal to or more than 10 years of age, the female children were mostly affected. In contrast, in children less than 5 years of age, male children were predominantly affected. A Korean study in 1999 showed similar results, although the number of cases was small9). The incidence of headache was higher and the duration of fever was longer in older children. Those results suggest that systemic symptoms other than the classic triad of fever, pharyngotonsillitis, and lymphadenopathy may be more frequent and more severe in older children and adolescents.
Mild transient elevations of hepatic aminotransferases are common in patients with EBV-associated IM1,11) and usually asymptomatic. Liver injury may be due to lymphocytic infiltration of the liver and proliferation of Kupffer cells15). In our study, the older age group had higher occurrence of liver enzyme elevation than the younger age group, although the level of ALT and AST was not elevated. The age related increase of liver enzymes may reflect a difference in the host immune response against EBV between infants and older children. In this study, all children with elevated levels of liver enzymes gradually recovered within a few weeks via supportive care.
Splenomegaly developed in the first three weeks of illness in at least 50% and hepatomegaly in about 30 to 50% of children with EBV-associated IM. In previous studies in Korea in 19947) and 19978), both splenomegaly and hepatomegaly were observed in about 42 to 70% of patients. The occurrence of hepatosplenomegaly was decreased in our study compared to previous studies. Our study showed 12.3% of patients with splenomegaly and 24.7% of patients with hepatomegaly.
IM often manifests with prolonged fever16,17). In this study, the mean duration of fever was 7.7 days, and, for prolonged fever, more than 10 days was detected in 12 (14.8%) patients. Only 46% of children older than 10 years of age showed tonsillar exudate. Therefore, if older children suffer from prolonged fever without any localizing sign, EBV-associated IM must be considered17).
The treatment of EBV-associated IM is supportive. Rest and symptomatic treatment are the mainstays of management. Fluid and a soft diet along with acetaminophen or ibuprofen will help ease the symptoms of pharyngitis and fever. Patients who have splenomegaly should be advised to avoid contact sports to prevent the rare possibility of splenic rupture1,3). In this study, all patients recovered with conservative treatment without significant complications.
In conclusion, this study showed that EBV-associated IM in Korean children still occurs mostly in young children less than 10 years of age, with a peak incidence at 3 to 7 years. The incidence of fever and headache, fever duration, the proportion of females to males, and liver enzyme elevation all showed positive association with advancing age in children with EBV-associated IM. When compared to previous Korean studies about 15 years ago, the age distribution was similar and the incidence of hepatosplenomegaly was lower in our study.
References
1. Jenson HB. Robert MK, Richard EB, Hal BJ, Bonita FS,Epstein-Barr virus. editors. Nelson textbook of pediatrics. 2007;18th ed. Philadelphia: Saunders, :1372–1376.

2. de-Thé G, Geser A, Day NE, Tukei PM, Williams EH, Beri DP, et al. Epidemiological evidence for causal relationship between Epstein-Barr virus and Burkitt's lymphoma from Ugandan prospective study. Nature 1978;274:756–761.


4. Grotto I, Mimouni D, Huerta M, Mimouni M, Cohen D, Robin G, et al. Clinical and laboratory presentation of EBV positive infectious mononucleosis in young adults. Epidemiol Infect 2003;131:683–689.



5. Hong CY, Lee HS, Henle W, Henle GE. Epstein-Barr virus antibody levels in koreans. J Korean Med Assoc 1977;20:425–428.
6. Oh SH, Lee YA, Moon WY, Ko TS, Park YS, Moon HN, et al. Prevalence of Epstein-Barr virus (EBV) antibody in Korean children. J Korean Pediatr Soc 1994;37:804–811.
7. Moon WY, Oh SH, Ko TS, Park YS, Moon HN, Hong CY, et al. Infectious mononucleosis in children. J Korean Pediatr Soc 1994;37:822–831.
8. Choi JS, Kim TH, Park HY, Lim SC. Clinical analysis of infectious mononucleosis. Korean J Otolaryngol-Head Neck Surg 1997;40:914–921.
9. Lee HS, Lee SH, Kwon SW, Kim KR, Hur YD. Clinical study of infectious mononucleosis. Korean J Bronchoesophagol 1999;5:22–29.
10. Chan CW, Chiang AK, Chan KH, Lau AS. Epstein-Barr virus-associated infectious mononucleosis in Chinese children. Pediatr Infect Dis J 2003;22:974–978.


11. Cheng CC, Chang LY, Shao PL, Lee PI, Chen JM, Lu CY, et al. Clinical manifestations and quantitative analysis of virus load in Taiwanese children with Epstein-Barr virus-associated infectious mononucleosis. J Microbiol Immunol Infect 2007;40:216–221.

12. Biggar RJ, Henle G, Böcker J, Lennette ET, Fleisher G, Henle W. Primary Epstein-Barr virus infections in African infants. II. Clinical and serological observations during seroconversion. Int J Cancer 1978;22:244–250.


13. Fleisher G, Henle W, Henle G, Lennette ET, Biggar RJ. Primary infection with Epstein-Barr virus in infants in the United States: clinical and serologic observations. J Infect Dis 1979;139:553–558.


14. Chan KH, Tam JS, Peiris JS, Seto WH, Ng MH. Epstein-Barr virus (EBV) infection in infancy. J Clin Virol 2001;21:57–62.


Table 1
Male-to-Female Ratio of Childhood Epstein-Barr Virus-Associated Infectious Mononucleosis by Age Group
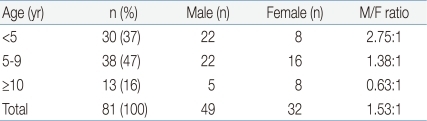
Table 2
Clinical Findings of Childhood Epstein-Barr Virus-Aassociated Infectious Mononucleosis by Age Group



 PDF Links
PDF Links PubReader
PubReader PubMed
PubMed Download Citation
Download Citation


