Article Contents
| Korean J Pediatr > Volume 54(12); 2011 |
Abstract
Hypoglossal nerve palsy (HNP) is an uncommon neurological abnormality that can provoke characteristic clinical signs, including unilateral atrophy of the tongue musculature. We present the case of a healthy 11-year-old Korean male who was admitted to the outpatient department of our institution with acute onset dysarthria, tongue fasciculations, and right-sided tongue weakness upon awakening. His evaluation included a virology work-up, neck magnetic resonance imaging (MRI), brain MRI, and otorhinolaryngological physical examination; all tests were normal and showed no evidence of inflammation. Fifteen days after the onset of symptoms, the patient recovered completely. Herein, we report a case of idiopathic isolated HNP in a Korean male.
The importance of the hypoglossal nerve, or cranial nerve XII, is often devalued; many times its description is reduced to a few words under "the last four cranial nerves," because damage to the 12th nerve rarely causes much inconvenience. Indeed, this nerve is considered a convenient "spare", suitable for transplantation surgery to reanimate a paralyzed face1). The motor composition of cranial nerve XII is highly complex and incompletely understood, and the nucleus consists of 4 topographically distinct subnuclear columns. Hypoglossal nerve palsy (HNP) is an uncommon neurological abnormality. Damage to this nerve produces characteristic clinical manifestations, including unilateral atrophy of the tongue musculature. The causes of HNP include known tumors (49%), trauma (12%), stroke (6%), hysteria (6%), surgery (5%), multiple sclerosis (5%), infection (4%), Guillain-Barre neuropathy (4%) and idiopathic (3%)2). This report describes a pediatric case of idiopathic isolated HNP with Solumedrol treatment for 12 days with complete recovery after 15 days.
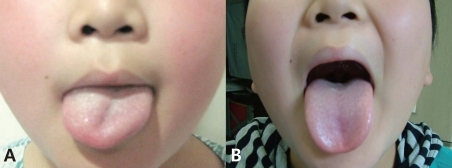
A 11-year-old Korean male was admitted to the outpatient department with sudden onset dysarthria, tongue fasciculations, and right-sided tongue weakness upon awakening (Fig. 1A).
His medical history included a normal spontaneous vaginal delivery at 39 weeks with a birth weight of 3,200 g. He had normal developmental milestones, his medical history was unremarkable, and his family history was non-specific. His vaccination history was complete. He had no recent history of upper respiratory track infection and had no snoring at night. There was no history of surgery or trauma.
Upon initial physical examination, his body temperature was 36.2℃, heart rate 72 beats/min and blood pressure 120/70 mm Hg. The neurological examination revealed right-sided deviation of his tongue upon protrusion, but cranial nerve function, cerebellar function and upper motor neuron function were non-specific; otherwise, physical exam findings were non-specific. A laboratory investigation, neck computed tomography (CT), brain magnetic resonance imaging (MRI) and otorhinolaryngology consultation were done. Laboratory evaluations revealed: hemoglobin 13.1 g/dL, hematocrit 37.5%, white blood cell 9,300/mm3, platelets 382,000/mm3, peripheral blood smear non-specific, sodium 139.1 mmol/L, potassium 3.9 mmol/L, GOT/GPT 27/23 IU/L and blood urea nitrogen/creatinine 10.1/0.6. Epstein-Bare virus (EBV) viral capsid antigen (VCA) immunoglobulin (Ig) M negative, Epstein-Barr virus nuclear antigen positive, EBV VCA IgG positive, Cytomegalovirus (CMV) IgM negative, CMV IgG negative.
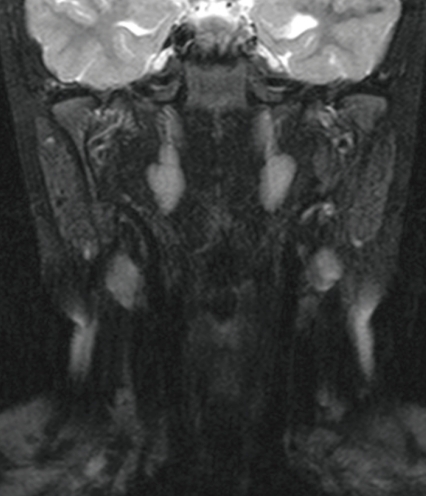
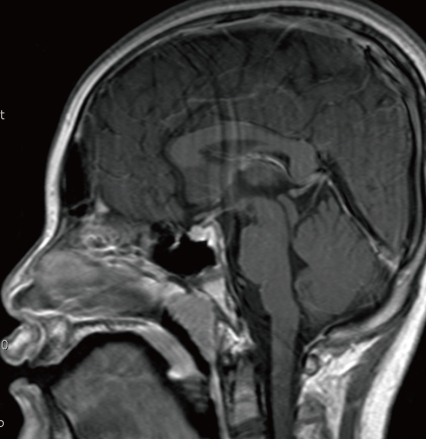
Neck MRI (Fig. 2) and brain MRI (Fig. 3) findings were normal. The otorhinolaryngology consultation disclosed non-specific anatomical problems and no evidence of inflammation.
The working diagnosis was that of idiopathic isolated HNP. The patient was treated with Solumedrol (60 mg/day #3) for 12 days. Fifteen days after symptom onset, the patient completely recovered (Fig. 1B).
The hypoglossal nerve is underrated, relegated to a few words under "the last 4 cranial nerves" in most neurology texts. Damage to the 12th nerve rarely causes much inconvenience; indeed, this nerve is often used in transplantation surgery to reanimate a paralyzed face. In addition to having complex afferent input within the brain stem and rich anastamoses in the neck, the hypoglossal nerve probably carries small proprioceptive and sympathetic contributions. Its motor composition is highly complex and incompletely understood, and the nucleus consists of 4 topographically distinct subnuclear columns1).
Keane2) published a review of 26 years of personal experience with cases of HNP in a large public hospital. He reported on 100 patients ranging in ages from 8 to 73 years; 51 cases were men and 49 cases were women. Thirty-one patients had right side HNP, 36 had left side, and 33 had HNP on both sides. He also reported that HNP usually appears as signs rather than symptoms. Tumors, predominantly malignant, produced nearly half of the palsies (49 cases). Trauma due to gunshots (12 cases) was the second most common cause. Stroke (6 cases), hysteria (6 cases), multiple sclerosis (6 cases), surgery (5 cases), Guillain-Barre neuropathy (4 cases), infection (4 cases), and idiopathic (3 cases) together accounted for about one third of the patients.
Boban et al.3) reported on 4 patients with isolated HNP due to different etiologies. The different etiologies were pseudoaneurysm of the right internal carotid artery, 2 aneurysms on the right and left middle cerebral artery, postinfectious acute disseminated encephalomyelitis, and metastatic tumor on the right ventrolateral surface of the medulla oblongata.
Combarros et al.4) reported 9 cases of isolated unilateral HNP. In 4 cases, no cause was found, and all the patients had an excellent outcome. In 3 cases, metastatic disease prompted the unilateral HNP. In the other 2 cases, the causes of isolated unilateral HNP were Chiari malformation and dural arteriovenous fistula of the transverse sinus.
Tommasi-Davenas et al.5) reported 32 cases of HNP. Seventeen cases were related to predominant malignant tumors. Six cases were of vascular origin, 4 cases were due to head or neck trauma, 4 cases were from various inflammatory processes, and 1 case was caused by Chiari's malformation.
Other case reports of HNP have revealed the causes to be multiple myeloma6), adenoid cystic carcinoma7), pyogenic meningitis8), a late complication of radiation therapy of head and neck carcinoma9), infectious mononucleosis10-11), a complication of post streptococcal infection12), possibly linked to influenza vaccination13), and multiple sclerosis14).
Giuffrida et al.15) reported 3 patients with isolated unilateral HNP who experienced an excellent outcome. In 2 cases, the patients had idiopathic HNP, and 1 patient was found to have right-sided occipital trauma. Giuffrida et al.15) believed that the occurrence of benign and idiopathic isolated unilateral HNP is more frequent than previously reported and proposed that no therapy is required in patients with HNP that is likely viral in origin. In the diagnosis of benign HNP, neuroimaging studies (X-ray, CT scan, MR, MR angiography) remain mandatory in order to exclude tumoral, spontaneous, traumatic, or vascular causes in view of the possibility of a specific or early anticoagulant treatment.
Our case represents idiopathic isolated HNP in a patient who completely recovered within 15 days. However, isolated HNP in children is often an ominous sign of underlying malignancy. Details regarding the patient's trauma and vaccination history were necessary. An MRI scan of the brain, brain stem and skull base is mandatory, and viral serology, particularly for EBV, were useful supplementary investigations.
References
1. Cushing H. The surgical treatment of facial paralysis by nerve anastomosis: with the report of a successful case. Ann Surg 1903;37:641–659.


3. Boban M, Brinar VV, Habek M, Rados M. Isolated hypoglossal nerve palsy: a diagnostic challenge. Eur Neurol 2007;58:177–181.


4. Combarros O, Alvarez de Arcaya A, Berciano J. Isolated unilateral hypoglossal nerve palsy: nine cases. J Neurol 1998;245:98–100.


5. Tommasi-Davenas C, Vighetto A, Confavreux C, Aimard G. Causes of paralysis of the hypoglossal nerve. Apropos of 32 cases. Presse Med 1990;19:864–868.

6. Tucker D, Musuka C. Isolated 12th cranial nerve palsy as a complication of multiple myeloma. Br J Haematol 2004;124:1


7. Adams H, Verhoff MA, Hagemeyer TP, Müller KM. Arachnoid cyst with consecutive brain stem atrophy, hypoglossal nerve paresis and tongue atrophy. Pathologe 2001;22:266–269.


8. Singh S, Shankar RR, Singhi PD, Kumar L. Isolated hypoglossal nerve palsy in pyogenic meningitis. Indian Pediatr 1988;25:705–706.

9. Cheng VS, Schultz MD. Unilateral hypoglossal nerve atrophy as a late complication of radiation therapy ofhead and neck carcinoma: a report of four cases and a review of the literature on peripheral and cranial nerve damages after radiation therapy. Cancer 1975;35:1537–1544.


10. Parano E, Giuffrida S, Restivo D, Saponara R, Greco F, Trifiletti RR. Reversible palsy of the hypoglossal nerve complicating infectious mononucleosis in a young child. Neuropediatrics 1998;29:46–47.


11. Wright GD, Lee KD. An isolated right hypoglossal nerve palsy in association with infectious mononucleosis. Postgrad Med J 1980;56:185–186.



12. Hadjikoutis S, Jayawant S, Stoodley N. Isolated hypoglossal nerve palsy in a 14-year-old girl. Eur J Paediatr Neurol 2002;6:225–228.


13. Felix JK, Schwartz RH, Myers GJ. Isolated hypoglossal nerve paralysis following influenza vaccination. Am J Dis Child 1976;130:82–83.





 PDF Links
PDF Links PubReader
PubReader PubMed
PubMed Download Citation
Download Citation


