Introduction
Proteinuria is defined as a condition where urinary protein level is greater than 150 mg/24-hour in adults, and greater than 4 mg/m2/hr in children. Chronic glomerular nephritis occurs when proteinuria continues for more than a 3 month period1). Proteinuria exists as several forms; there is glomerular proteinuria that is caused by loss of the function of the glomerular barrier, and tubular proteinuria that is caused by failure of resorption of protein that filtrates in the gluomerulus2). The kidney diseases that chief symptom is proteinuria have different patholgical findings; in nephrotic syndrome, glomerular injury appears in the early period of nephrotic syndrome, and progressive tubular injury appears in late period, and in tubulointerstitial disease, tubular dysfunction appears in the early period, and secondary glomerular injury appears in the late period3).
The urinary excretion of β2-microglobulin (β2-M) and N-acetyl-β-D-glucosaminidase (NAG) has been used as a marker of renal tubular cell injury. β2-M is a small circulating protein with a molecular weight of 11,800, which is partly filtrated at the glomerulus. After glomerular filtration, β2-M is normally 99.9% reabsorbed in proximal tubule and degradated in the lysosome of the renal tubular cell, and finally it is excreted in the urine4,5). In tubular disorders, this mechanism is impaired, and urinary excretion of β2-M is increased. NAG is one of the low molecular weight proteins found in high concentrations in the lysosomes of renal proximal tubules. The increase of urinary excretion of NAG usually implies there is renal tubular injury6).
Glomerular filtration rate (GFR) is widely used as the best measurement of kidney function. Serum creatinine is most commonly used to estimate GFR because it can be filtrated through glomerular basement membrane freely, and is not reabsorbed in the proximal tubule7,8). The creatinine clearance is measured by serum creatinine using the Schwartz method (K [coefficient]×[height/serum creatinine])9). It may also be measured by the Bokencamp method (137/cystatin C-20.4)10). The decrease of GFR indicates the glomerular injury, and the increase of excretion of tubular enzyme indicates the tubular injury. As the kidney disease is chronic and the proteinuria continues, the glomerular injury can have an effect on the tubular injury; the increase of the protein that is filtrated through the glomerulus can have an effect on reabsorption of the protein in the renal tubule. We hypothesize that evaluation of the urinary tubular enzyme can be helpful to predict kidney function in chronic glomerular disease.
In this study, we evaluated the association of the GFR and urinary β2-M and urinary NAG in children with chronic kidney disease, and we evaluated glomerular function estimated by urinary β2-M and urinary NAG measurement in chronic kidney disease.
Materials and methods
1. Study population
Fifty-two children with various chronic glomerular disease, who had continuous proteinuria (≥4 mg/m2/hr) for more than 3 months, were examined at the Department of Pediatric Nephrology at Chung-Ang University Hospital, Seoul between Janunary 2003 and August 2009. From the group 28 patients were male and 24 female, the mean age was 8.1 years, and the mean body weight was 30.6 kg.
Of these 52 patients, 13 children were diagnosed with minimal change disease, 3 children diagnosed with focal segmental glomerulosclerosis, 17 children with immunoglobulin A nephropathy, 15 children with Henoch-Schönlein purpura nephritis, 3 children with poststreptococcal glomerulonephritis, and 1 child was diagnosed with thin glomerular basement membrane disease (Table 1).
Nineteen patients were categorized with stage 1 chronic kidney disease, 27 patients were stage 2, 5 patients were stage 3, and 1 patient was stage 4 (Table 2).
2. Materials
We obtained urinary samples in the early morning and investigated the 24-hour urinalysis and the hematologic values in all of 52 patients. Serum creatinine, creatinine clearance, serum cystatin C, urinary β2-M and urinary NAG were measured in these patients.
The venous blood samples were kept in a freezer at -20℃. Twenty-four hours urinary samples were kept in a freezer at -20℃, and we added 1.0 NaOH to the urine samples to prevent β2-M degradation in the acidic urine. We measured β2-M by radioimmunoassay using Coat A-Conunt Beta-2 Microglobulin IRMA kit (Diagnostic Products Co., Los Angeles, CA, USA). Serum cystatin C was measured by the particle enhanced nephelometric immunoassay (Dade Behring Marburg GmbH, Marburg, Germany).
We investigated the association of the GFR and the urinary β2-M and the urinary NAG in patients with proteinuria. GFR was estimated by measuring creatinie clearance using both Schwartz and Bokencamp methods. We also evaluated the association of the creatinine clearance and the urinary β2-M and the urinary NAG at the each stage of chronic kidney disease.
Results
1. Correlation between GFR and urinary NAG in children with chronic glomerular disease
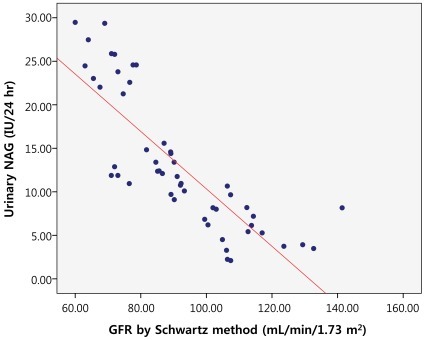
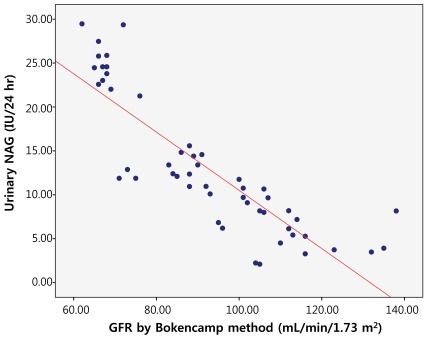
We evaluated the correlation between the GFR and the urinary NAG by measuring creatinine clearance using both Schwartz and Bokencamp methods. There was a negative correlation between the creatinine clearance and the 24-hour urinary NAG in all patients with chronic proteinuria (r=-0.817; P<0.01) (Fig. 1). AS well there was a negative correlation between the 24-hour urinary NAG and the GFR by Schwartz method (r=-0.821; P<0.01) (Fig. 2). We found a negative correlation between the 24-hour urinary NAG and the GFR by Bokencamp method (r=-0.858; P<0.01) (Fig. 3).
2. Correlation between GFR and urinary β2-M in children with chronic glomerular disease
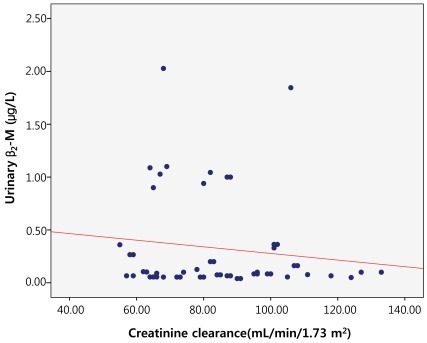
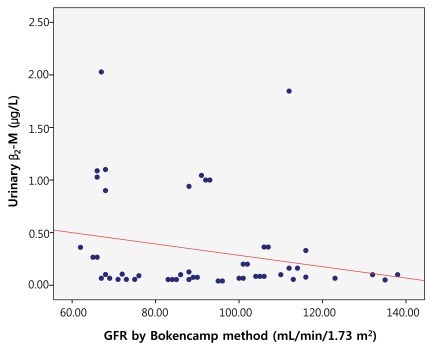
The correlation between GFR and urinary β2-M using creatinine clearance was evaluated using both Schwartz and Bokencamp methods. There was no correlation between the creatinine clearance and the 24-hour urinary β2-M among the patients with chronic proteinuria (r=-0.132; P=0.352) (Fig. 4). There was also no correlation between the 24-hour urinary β2-M and the GFR by Schwartz method (r=-0.123; P=0.386) (Fig. 5). Additionally, there was no correlation between the 24-hour urinary β2-M and the GFR by Bokencamp method (r=-0.231; P=0.099) (Fig. 6).
3. Correlation between the creatinine clearance with respect to urinary NAG and urinary β2-M in the children with chronic glomerular disease
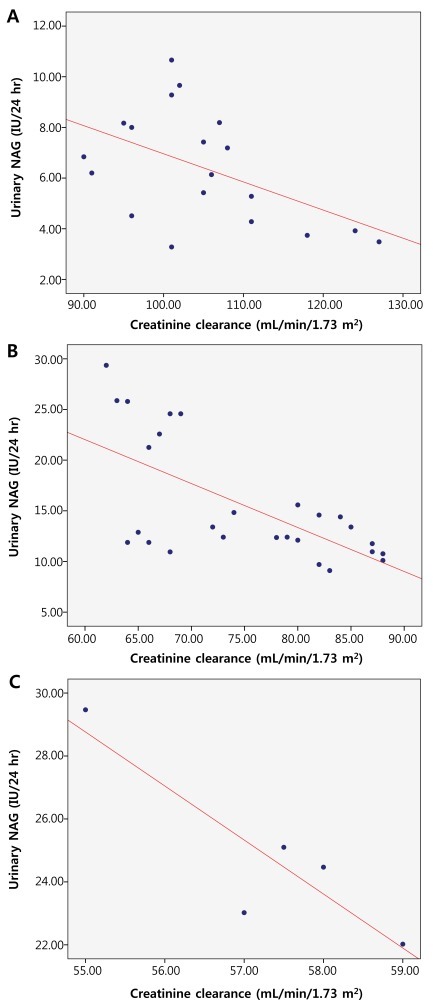
We evaluated the correlation between the GFR and the urinary NAG as well as with urinary β2-M using creatinine clearance in each stage of chronic kidney disease. In stage 1 chronic kidney disease, there was a negative correlation between the creatinine clearance and the 24-hour urinary NAG (r=-0.503; P=0.028). In stage 2 chronic kidney disease, there was a negative correlation between the creatinine clearance and the 24-hour urinary NAG (r=-0.651; P<0.01). With regards to stage 3 chronic kidney disease, there was again a negative correlation between the creatinine clearance and the 24-hour urinary NAG (r=-0.888; P=0.044) (Fig. 7). But, in stage 1 chronic kidney disease, there was no correlation between the creatinine clearance and the 24-hour urinary β2-M (r=-0.017; P=0.945). In stage 2 chronic kidney disease, there was no correlation between the creatinine clearance and the 24-hour urinary β2-M (r=-0.020; P=0.922), as was also the case in stage 3 chronic kidney disease, where there was no correlation between the creatinine clearance and the 24-hour urinary β2-M (r=-0.632; P=0.253) (Fig. 8).
Discussion
In this study, there was a negative correlation between the creatinine clearance and the 24-hour urinary NAG and no correlation between the creatinine clearance and the 24-hour urinary β2-M in all patients with chronic proteinuria. These results are consistent when they are compared using both Schwartz and Bokencamp methods for evaluation. In each stage of chronic kidney disease, there was a negative correlation between the creatinine clearance and the 24-hour urinary NAG and no correlation between the creatinine clearance and the 24-hour urinary β2-M.
Among the several methods for evaluating the kidney function, the calculation of GFR is one of the most common methods. GFR is the measure of clearance of inulin, iohexol, Cr-ethylenediaminetetraacetic acid, TC-diethylene triamine penta-acetic acid and I-iothalamate, which are extracorporeal materials, and is also the measure of the clearance of serum creatinine, blood urea nitrogen and β2-microglobulin, which are body materials. In the clinical field, serum creatinine is the most commonly used because of several advantages: it can filtrate through the glomerulus freely and is not reabsorbed in the proximal tubule, and additionally it has cost effectiveness7,8). In this study, the creatinine clearance is represented by serum creatinine measurement, and evaluated by Schwartz method (K [coefficient] × [height/serum creatinine])9). It can also be measured by the Bokencamp method (137/cystatin C-20.4)10). Cystatin C is a non-glycosylated protein which consists of 122 amino acids. It is synthesized in nucleated cells, and can filtrate through the glomerular basement membrane freely and is reabsorbed in the proximal tubule. Thus, the serum concentration of cystatin C is dependent on the GFR11,12).
There are many studies for proteins and enzymes as markers for early detection of renal tubular injury; β2-M, retinol-binding protein, alpha1-microglobulin, NAG13).
Bazzi et al.14) had studied urinary NAG as a marker of tubular injury. They measured the urinary NAG of patients with primary glomerulonephritis, and concluded that urinary NAG excretion could be considered as a marker of tubular damage in the early stage of glomerulonephritis, and might be an effective non-invasive test for assessing the damage. NAG is a low molecular weight protein and is found in high concentrations in the lysosomes of renal proximal tubules. The increase of urinary excretion of NAG means that there is renal tubular injury. NAG, especially the isoenzyme A, is in the lysosome of the renal tubular cell, and most urinary NAG comes from the renal tubular cell6). The urinary NAG has been used as a marker of renal tubular cell injury, and it increases in glomerular nephritis, diabetes mellitus nephritis, cadmium poisoning, radiographic agent nephropathy, chronic pyelonephritis15-17).
In our study, we measured the level of urinary NAG in patients with glomerulonephritis, and evaluated the correlation between the urinary NAG and GFR. We found that as the glomerular nephritis was progressive, where GFR is typically lower, the excretion of urinary NAG increased. Our results are similar to Bazzi's study.
According to Piscator13), the urinary β2-M is a good marker for early detection of proximal tubular damage. β2-M is isolated from the urine of patient with Wilson's disease and chronic cadmium poisoning by Berggard and Bearn18) in 1986. β2-M is naturally made in most nucleated cells, especially lymphocytes, and it exists in serum, urine, cerebrospinal fluid, saliva, and ascites19,20). β2-M is a small circulating protein with a molecular weight of 11,800, which is partly filtrated at the glomerulus. After glomerular filtration, β2-M is normally 99.9% reabsorbed in the proximal tubule and degradated in the lysosome of the renal tubular cell, and finally excreted in the urine4,5). The excretion of urinary β2-M dose not always increase when the serum β2-M increases, and it can increase when the reabsorption decreases due to dysfunction of renal proximal tubule; cadmium poisoning, mercury poisoning, Balkan nephropathy, tubule injury due to antibiotics, acute glomerular nephritis, renal failure, urogenital malformation21,22). In rheumatic arthritis, systemic lupus erythematosis, Crohn's disease, chronic liver disease, leukemia, and lymphoma, the concentration of serum β2-M increases. If these disorders do not exist, the concentration of serum β2-M is dependent on the GFR, and the excretion of urinary β2-M is dependent on the function of the proximal renal tubule23,24).
In our study, we measured the level of the urinary β2-M in patients with glomerulonephritis, and evaluated the correlation between the urinary β2-M and the GFR. We found that there was no correlation between urinary β2-M and GFR, which was a different result in relation to urinary NAG although both urinary NAG and urinary β2-M are considered markers of tubular injury.
In Sherman's study, both urinary NAG and urinary β2-M increased in patients with progressive glomerulonephritis. Sherman et al.17) had shown that there was a tubulointerstitial involvement in the renal parenchymal diseases. Our results of study about urinary β2-M is different from that of Sherman's study.
Chiaramonte had conducted studies regarding urinary NAG and urinary β2-M in patients with renal disease. He found that both urinary NAG and urinary β2-M increased further in the progressive renal disease, and he concluded that these were useful and accurate markers of renal injury progression23). In our study, we evaluated urinary NAG and urinary β2-M in each of the three stages of renal disease, which was classified by GFR. Chronic kidney disease is classified into five stages by GFR. Stage 1 is characterized by slightly diminished function with normal or relatively high GFR (>90 mL/min/1.73 m2). In stage 2 there is mild reduction in GFR (60 to 89 mL/min/1.73 m2), stage 3 consists of moderate reduction in GFR (30 to 59 mL/min/1.73 m2), stage 4 has a severe reduction in GFR (5 to 29 mL/min/1.73 m2), and stage 5 is established kidney failure (<15 mL/min/1.73 m2)1). In our study, we found that as the stage was higher, the correlation between urinary NAG and GFR was a more steep negative coefficient of correlation.
Based on our findings, we believe that the urinary NAG can be used to predict the function of the glomerulus in chronic kidney disease. We also suggest further investigation is necessary in larger patient populations, along with follow-up studies. As well, further studies to investigate the differences between urinary NAG and urinary β2-M as evaluation markers will be necessary.








 PDF Links
PDF Links PubReader
PubReader PubMed
PubMed Download Citation
Download Citation


