Article Contents
| Korean J Pediatr > Volume 58(5); 2015 |
|
Abstract
Purpose
The purpose of this study was to determine the frequency of CD4+CD25+FoxP3+ regulatory T cells (Treg) in the peripheral blood of patients with childhood chronic immune thrombocytopenic purpura (ITP) exhibiting thrombocytopenia and spontaneous remission. The findings of this study indicate the possibility of predicting spontaneous recovery and pathogenesis of childhood chronic ITP.
Methods
Eleven children with chronic ITP (seven thrombocytopenic and four spontaneous remission cases; mean age, 8.8 years; range, 1.7-14.9 years) were enrolled in this study. Five healthy children and eight healthy adults were included as controls. The frequency of Treg was evaluated by flow cytometry in the peripheral blood.
Results
In this study, four patients (36%) achieved spontaneous remission within 2.8 years (mean year; range, 1.0-4.4 years). The frequency of Treg was significantly lower in patients with persisting thrombocytopenia (0.13%±0.09%, P<0.05), than that in the patients with spontaneous remission (0.30%±0.02%), healthy adults controls (0.55%±0.44%), and healthy children controls (0.46%±0.26%). A significantly positive correlation was found between the frequency of Treg and the platelet count in children.
Conclusion
These data suggest that a lower frequency of Treg contributes to the breakdown of self-tolerance, and may form the basis for future development of specific immunomodulatory therapies. Furthermore, Treg frequency has prognostic implication toward the natural course and long-term outcomes of childhood chronic ITP.
To maintain the immune tolerance and to prevent autoimmune disease, CD4+CD25+ FoxP3+ regulatory T cells (Treg), CD4+ T cells with high expression of CD25, and transcription factor forkhead box P3 (FoxP3), which is also named as FoxP3 regulatory T cells, play a fundamental role. Decreased number of Tregs and impairment of Tregs function have been reported in patients with various autoimmune diseases, such as systemic lupus erythematosus, rheumatic arthritis, multiple sclerosis, and diabetes as well as immune thrombocytopenic purpura (ITP)1,2,3,4).
Childhood ITP is one of the most common benign hematologic disorders which is characterized by the isolated, immune-mediated thrombocytopenia and mucocutaneous bleeding. Childhood ITP is usually a self-limiting disease and is typically normalized within several months. However, approximately 20%-30% of children have persistent thrombocytopenic states for more than 6-12 months, which is called the chronic ITP with increasing risk of bleeding5,6,7).
Chronic ITP is considered to be a pathogenically and clinically heterogeneous disease involving multifactorial autoimmune mechanisms of both humoral and cellular immunity8,9,10,11). It is not easy to predict whether the newly diagnosed ITP is self-limiting or persisting and whether the thrombocytopenia can be recovered during follow-up period.
Fortunately, even in chronic ITP, spontaneous remission can occur within 5 years after diagnosis, in 30% to 60% of childhood chronic ITP patients12). Several studies on the outcome of childhood chronic ITP have described some clinical prognostic factors. However, there is little information about the laboratory immunologic markers associated with spontaneous remission in chronic ITP. If the predictors or indicators of spontaneous remission and natural course in chronic childhood ITP can be confirmed, it will improve treatment strategies and quality of life as well as preventing unnecessary splenectomy and treatment cost for physicians, patients, and parents13,14,15).
Chronic ITP is considered to be a pathogenically and clinically heterogeneous disease involving multifactorial autoimmune mechanisms of both humoral and cellular immunity8,9).
Recently, there have been increasing amount of evidences indicating that an impairment of regulatory T cells plays a critical role in pathogenesis of ITP16). However, there is little information about the role of Tregs for the patients with spontaneous remission from chronic ITP, compared to the persisting chronic ITP.
The objective of this study is to investigate the frequency of Treg in the peripheral blood of the childhood chronic ITP with thrombocytopenia and spontaneous remission. The findings in this study would elucidate the possibility of predicting the spontaneous recovery and pathogenesis of childhood chronic ITP.
A prospective cohort analysis was conducted from the data of pediatric patients with chronic ITP, who were referred to our department during the past 7 years. In accordance with Institutional Review Board procedures at Chungbuk National University Hospital (2008-07-022), data from the medical records were analyzed after obtaining informed consents from the parents of each participant, prior to the study. A total of 11 patients with chronic ITP were enrolled in this study.
During the study period, all patients with chronic ITP were followed up with the intervals of 1-3 months, after 1 year from initial diagnosis. Finally, all chronic ITP patients were divided into two groups as follows: persistent group and spontaneous remission group.
Chronic ITP was defined as having the persisting isolated thrombocytopenia for more than 12 months and platelet count of less than 150,000/mm3, which was used as the threshold for ITP diagnosis. All of the patients had primary ITP without any obvious initiating or underlying cause. Spontaneous remission was defined as having the platelet counts remaining above 150,000/mm3 for more than 6 months and/or 3 serial tests without ongoing treatment during follow-up.
Seven chronic ITP patients were allocated to persistent group (CITP-P) and the other four patients to spontaneous remission group of chronic ITP (CITP-SR). At the time of sampling, all patients had not received steroid or immunoglobulin therapy for at least 1 month.
Five healthy children and eight healthy adults were included in this study as controls. The following information was collected from the patients and controls at the time of their enrollment: sex, age at the enrollment and diagnosis, follow-up period, duration of disease, platelet count at the enrollment, and progress.
For blood sampling, 3 mL of peripheral venous blood was drawn into ethylenediaminetetraacetic acid tube for the complete blood count and flow cytometric analysis for the frequency of Treg. Treg was characterized by staining, using fluorescein isothiocyanate mouse antihuman CD4, allophycocyanin mouse antihuman CD25, and phycoerythrin mouse antihuman FoxP3 antibody (BD Pharmingen, San Diego, CA, USA) according to the manufacturer's instructions. Flow cytometry was performed using a FACScalibur cytometer and CELLQUEST software (BD biosciences, San Jose, CA, USA).
All statistical analyses were performed using SPSS ver. 17.0 (SPSS Inc., Chicago, IL, USA). Descriptive statistics were performed, including the mean and standard deviation for quantitative variables and number and percentage for qualitative variables. Normality was assessed by Shapiro-Wilk test. In pairwise comparison, Student t test were used for data fulfilled normal distribution. The Spearman rank correlation test was used to discover the strength of a link between two variables, for data did not fulfill normal distribution. In all tests, the level of significance was set at P<0.05.
Demographic and clinical data of all patients and healthy controls in this study are shown in Table 1. Among the enrolled 11 children with chronic ITP, the mean age at the time of this study was 8.78 years (1.75-14.89 years) and the mean age at initial diagnosis was 5.61 years (0.65-12.45 years). The mean duration of disease at the time of this study was 3.01 years (1.00-4.50 years). Compared to healthy children in the control group, the age and sex ratio of all enrolled patients were not significantly different from the total chronic ITP patients.
During this study, 4 patients (36%) achieved spontaneous remission (Fig. 1). For the 4 CITP-SR patients, the mean age at the time of this study was 7.30 years (1.75-10.84 years) and the mean age at the initial diagnosis was 4.13 years (0.65-7.79 years). The mean duration of disease, which indicates the period between diagnosis and spontaneous remission, was 2.78 years (1.00-4.40 years) and the mean follow-up period was 3.20 years (1.70-5.10 years). The mean platelet count of CITP-SR patients, at the time of this study, was 165.0×103/mm3 (155-175×103/mm3) (Table 1).
For the 7 CITP-P patients, the mean age at the time of this study was 9.62 years (3.50-14.89 years), and the mean age at the initial diagnosis was 6.45 years (1.56-12.45 years). The mean follow-up period, which also indicates the disease duration, was 3.14 years (1.90-4.50 years). The mean platelet count of CITP-P patients, at the time of this study, was 70.1×103/mm3 (29-133×103/mm3).
The frequency of Tregs in the patient subgroups and controls are shown in Table 2. The mean frequency of Tregs in the 7 CITP-P patients was 0.13%±0.09% (0.00%-0.26%). It was significantly lower than that of CITP-SR (0.30%±0.02%, 0.26%-0.31%), healthy adults controls (0.55%±0.44%, 0.09%-1.50%), and healthy children controls (0.46%±0.26%, 0.23%-0.86%) (P<0.05). However, the frequency of Tregs in CITP-SR patients appeared to have decreased, compared to that of adults and children controls, but no statistical significance was shown (P=0.73, P=0.214). Among the healthy controls, the frequency of Treg in children controls seemed to be higher than that of adults controls; but, there was no statistical significance (P=0.943).
For the total of 11 enrolled children with chronic ITP, the mean frequency of Tregs was 0.19%±0.11% (0.00%-0.31%). It was significantly lower compared to the frequency of healthy adults in the control group (0.55%±0.44%, 0.09%-1.50%, P<0.05). However, compared to that of healthy children in the control group (0.46%±0.26%, 0.23%-0.86%), the frequency of Tregs in the total chronic ITP patients appeared to have decreased, but there was no statistical significance (P=0.052).
A significantly positive correlation was found between the frequency of Treg and the platelet count in children (r=0.732, P=0.01) (Fig. 2). Other parameters such as sex, age at diagnosis, follow-up period, and duration of disease were not statistically correlated with the frequency of Tregs.
In this study, four patients (36%) achieved spontaneous remission within 2.8 years (1-4.4 years) after being diagnosed as ITP. From this result, it should be noted that the children with chronic ITP had a higher chance of spontaneous remission, even after the critical diagnosis; therefore, invasive therapy should be delayed. Several retrospective studies have reported that the overall spontaneous remission rate was from 30% to 80% with a follow-up period of up to 8 years, in the childhood chronic ITP patients12,15,17).
There are few studies assessing the prognostic factors for predicting the outcome of ITP after diagnosis. Jayabose et al.14) reported that the spontaneous remission rate was 56%, and the clinical factors such as age, gender, initial platelet count, initial treatment, and response to treatment did not have any prognostic significance. Although it is important to investigate the characteristics of clinical features in the chronic ITP, we need to establish the optimal immunologic and laboratory predictors.
For the mechanisms of chronic ITP, there are increasing amount of evidences indicating that an impairment of regulatory T cells plays a critical role in the pathogenesis of ITP18). Many studies have demonstrated a decreased frequency of Tregs or imbalance of circulating Th cell-associated cytokines in the adult ITP, which suggests the loss of peripheral immune tolerance. However, some of the studies have failed to detect any differences in Treg frequencies of adults ITP patients, compared to healthy controls16,19). These inconsistent results may mainly be due to the different phenotypes for Tregs according to investigators, the difficulty of methodology standardization, and the small accessible amount of Tregs which makes the study in children more difficult.
In the growing children, the role of Tregs is not fully elucidated; and there have been few studies related to predicting the natural course of the childhood chronic ITP1,3,19,20).
As the possible immunologic predictors, Treg was investigated in this study. The frequency of Tregs in CITP-P was significantly lower than that of CITP-SR or healthy adults and children controls, and a significantly positive correlation was found between the frequency of Treg and the platelet count in children. This suggests that the decreased frequency of Tregs is associated with the loss of immune tolerance for maintaining the platelet count, and recovery from the impairment of Tregs to normal level is associated with the spontaneous remission. However, there is no information on the normal levels of Tregs in children.
This study has several limitations. Our study was small-numbered and was conducted only at one point test. Further prospective cohort studies with immunologic and molecular investigations are needed to confirm our results.
The classic definition of chronic ITP is having a persistent thrombocytopenia for more than 6 months. Recently, the International Childhood ITP Study Group, American Society of Hematology guideline, and the international consensus statement have suggested that the criteria for the time of diagnosing chronic ITP should be reset to 12 months21,22,23). However, our result, as well as others studies, have shown that some patients achieved the spontaneous remission 1 year after diagnosis. Therefore, it is the authors' opinion that since the chronic ITP ranges from newly diagnosed to spontaneous recovered or persisting thrombocytopenia, the persisting ITP should be used instead of the chronicity definition.
In conclusion, these data suggest that the low frequency of Treg would contribute to the breakdown of self-tolerance as well as forming a base for the specific immunomodulatory therapies of the future. Furthermore, they will have prognostic significance toward the natural course and long-term outcome of the childhood chronic ITP.
Conflicts of interest
Conflicts of interest:
No potential conflict of interest relevant to this article was reported.
References
1. Buckner JH. Mechanisms of impaired regulation by CD4(+)CD25(+)FOXP3(+) regulatory T cells in human autoimmune diseases. Nat Rev Immunol 2010;10:849–859.




2. Ochs HD, Gambineri E, Torgerson TR. IPEX, FOXP3 and regulatory T-cells: a model for autoimmunity. Immunol Res 2007;38:112–121.


3. Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell 2008;133:775–787.


4. Yazdanbakhsh K, Zhong H, Bao W. Immune dysregulation in immune thrombocytopenia. Semin Hematol 2013;50(Suppl 1): S63–S67.



5. Cuker A, Cines DB. Immune thrombocytopenia. Hematology Am Soc Hematol Educ Program 2010;2010:377–384.


6. Imbach P, Kuhne T, Muller D, Berchtold W, Zimmerman S, Elalfy M, et al. Childhood ITP: 12 months follow-up data from the prospective registry I of the Intercontinental Childhood ITP Study Group (ICIS). Pediatr Blood Cancer 2006;46:351–356.


7. Bennett CM, Tarantino M. Chronic immune thrombocytopenia in children: epidemiology and clinical presentation. Hematol Oncol Clin North Am 2009;23:1223–1238.


9. Stasi R, Evangelista ML, Stipa E, Buccisano F, Venditti A, Amadori S. Idiopathic thrombocytopenic purpura: current concepts in pathophysiology and management. Thromb Haemost 2008;99:4–13.


10. Lazarus AH, Semple JW, Cines DB. Innate and adaptive immunity in immune thrombocytopenia. Semin Hematol 2013;50(Suppl 1): S68–S70.



11. Johnsen J. Pathogenesis in immune thrombocytopenia: new insights. Hematology Am Soc Hematol Educ Program 2012;2012:306–312.


12. Rosthoj S, Rajantie J, Treutiger I, Zeller B, Tedgard U, Henter JI, et al. Duration and morbidity of chronic immune thrombocytopenic purpura in children: five-year follow-up of a Nordic cohort. Acta Paediatr 2012;101:761–766.


13. Heitink-Polle KM, Nijsten J, Boonacker CW, de Haas M, Bruin MC. Clinical and laboratory predictors of chronic immune thrombocytopenia in children: a systematic review and meta-analysis. Blood 2014;124:3295–3307.


14. Jayabose S, Levendoglu-Tugal O, Ozkaynkak MF, Visintainer P, Sandoval C. Long-term outcome of chronic idiopathic thrombocytopenic purpura in children. J Pediatr Hematol Oncol 2004;26:724–726.


15. Yacobovich J, Revel-Vilk S, Tamary H. Childhood immune thrombocytopenia: who will spontaneously recover? Semin Hematol 2013;50(Suppl 1): S71–S74.


16. Andersson PO, Stockelberg D, Jacobsson S, Wadenvik H. A transforming growth factor-beta1-mediated bystander immune suppression could be associated with remission of chronic idiopathic thrombocytopenic purpura. Ann Hematol 2000;79:507–513.


17. Shim YJ, Kim UH, Suh JK, Lee KS. Natural course of childhood chronic immune thrombocytopenia using the revised terminology and definitions of the international working group: a single center experience. Blood Res 2014;49:187–191.



18. Godeau B. Immune thrombocytopenic purpura: major progress in knowledge of the pathophysiology and the therapeutic strategy, but still a lot of issues. Presse Med 2014;43(4 Pt 2): e47–e48.


19. Nishimoto T, Kuwana M. CD4+CD25+Foxp3+ regulatory T cells in the pathophysiology of immune thrombocytopenia. Semin Hematol 2013;50(Suppl 1): S43–S49.


20. Olsson B, Andersson PO, Jernas M, Jacobsson S, Carlsson B, Carlsson LM, et al. T-cell-mediated cytotoxicity toward platelets in chronic idiopathic thrombocytopenic purpura. Nat Med 2003;9:1123–1124.


21. Provan D, Stasi R, Newland AC, Blanchette VS, Bolton-Maggs P, Bussel JB, et al. International consensus report on the investigation and management of primary immune thrombocytopenia. Blood 2010;115:168–186.


Fig. 1
Platelet count of patients with CITP-P (A) and CITP-SR (B) during the follow-up period. Steroid or immunoglobulin therapy was given to patients with severe thrombocytopenia and bleeding. ITP, immune thrombocytopenic purpura; CITP-P, chronic ITP with persisting thrombocytopenia; CITP-SR, chronic ITP with spontaneous remission.
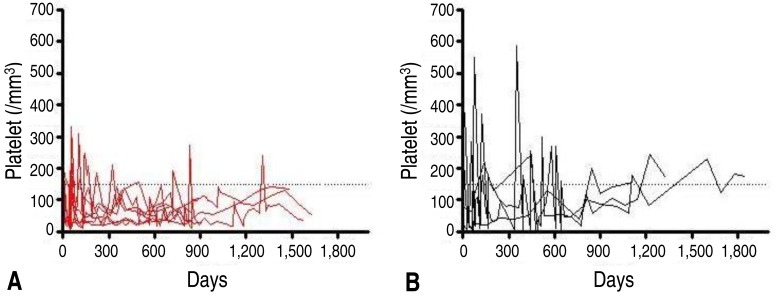
Fig. 2
Correlation between Treg frequencies and platelet count among all children (r=0.732, P=0.01). Treg, CD4+ CD25+ FoxP3+ regulatory T cell.
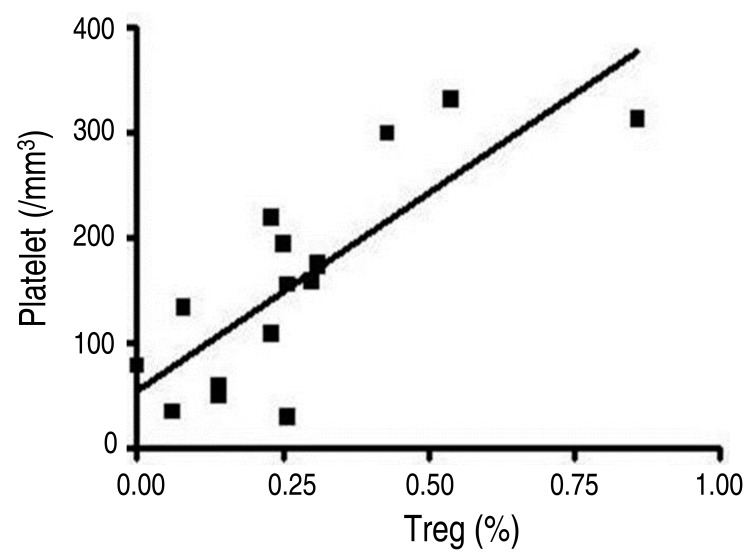
Table 1
Patient group details and Treg frequency
Table 2
Comparison between patient groups (CITP-P and CITP-SR) and control subjects
Values are presented as mean±standard deviation.
ITP, immune thrombocytopenic purpura; CITP-P, chronic ITP with persisting thrombocytopenia; CITP-SR, chronic ITP with spontaneous remission; PLT, platelet count; Tregs, CD4+CD25+FoxP3+ regulatory T cells.
*Significance compared to CITP-SR, P<0.05. †Significance compared to healthy children controls, P<0.05. ‡Significance compared to healthy adults controls, P<0.05.



 PDF Links
PDF Links PubReader
PubReader PubMed
PubMed Download Citation
Download Citation


