Article Contents
| Korean J Pediatr > Volume 58(5); 2015 |
|
Abstract
Purpose
Mycoplasma pneumoniae (MP) pneumonia epidemics have occurred in 3- to 4-year cycles in Korea. We evaluated the epidemiologic characteristics of MP pneumonia in Daejeon, Korea, from 2003 to 2012.
Methods
We retrospectively analyzed 779 medical records of children (0-15 years of old) with MP pneumonia admitted to our institution and compared the data from 3 recent epidemics.
Results
In 779 patients, the mean age and male-to-female ratio were 5.0±2.2 years and 1:1, and most cases were observed in autumn. There were three epidemics during the study period, in 2003, 2006-2007, and 2011. In our comparison of the three epidemics, we found no differences in mean age, the male-to-female ratio, hospital stay, or the rate of seroconverters during hospitalization. All three epidemics began in early summer and peaked in September 2003 and 2011 and in October 2006 and then gradually decreased until the next year's spring season, although the 2006 epidemic extended further into 2007. The peak age groups in the children in 2003 and 2006 were 3-6 year-olds (57.5% and 56%, respectively), but in the 2011 epidemic, the peak group was 1-4 year-olds (46.5%). The proportion of the <2 years of age group was 20%, 15.7% and 28.8%, and >10 years of age group was 5.2%, 13.8%, and 14.8% of total patients, respectively.
Mycoplasma pneumoniae (MP) is an important pathogen of respiratory diseases from mild upper respiratory tract infections to severe fatal pneumonia in children and young adults1,2). MP pneumonia has been reported in 10%-40% of cases of community acquired pneumonia, and its incidence is higher during epidemics. MP pneumonia might be endemic in large societies globally; an epidemic occurs every 3-7 years3,4,5,6,7,8). In Korea, MP epidemics have been reported every 3-4 years since the early 1980s, and there have been some epidemiologic changes, including the age of predominance between recent and past epidemics9,10,11). Epidemiological findings of MP infection, including age distribution of the patients and the cycle of epidemic, reportedly differ according to the populations that may have different population density, socioeconomic condition, and medical environment. However, the main clinical characteristics of MP infection, such as being self-limited with various phenotypes and the immunopathogenesis of pneumonia, might be same across populations.
In this study, we evaluated patients with MP pneumonia for the decade from 2003 to 2012 and compared epidemiological data from 3 MP pneumonia epidemics during the study period with more than 100 patients a year.
We retrospectively analyzed the data of 779 children (0-15 years of age) with MP pneumonia who were admitted to The Catholic University of Korea, Daejeon St. Mary's Hospital from January 2003 to December 2012. The selection of children with MP pneumonia was based on 2 times examinations of the antimycoplasma IgM antibody: 2 times examinations of titer (Serodia-Myco II, Fujirebio Inc., Tokyo, Japan; positive cutoff value≥1:40) and cold agglutinin test titer (positive cutoff value ≥1:32). The subjects were selected if antimycoplasma antibody titers showed a seroconversion (from negative to positive of the cutoff values) or increased antibody titers (≥4 folds) with corresponding cold agglutinin titers (including seroconversion or increased antibody titer) were detected during hospitalization. Patients who were positive in both assays at admission and discharge, but had decreased titers at discharge were excluded from the study, regarded as having a recent past infection during the epidemic period. In this study, we defined an epidemic as >100 patients per year and 4 folds more patients than in the previous year.
The written agreement was obtained by parents or guardians of all patients at admission for their clinical records to be used in future study. The study was approved by the Institutional Review Board of the Catholic University of Korea, Daejeon St Mary's Hospital.
All calculations were performed with SPSS ver. 14.0 (SPSS Inc., Chicago, IL, USA). Comparisons between groups were performed using the Student t-test and the analysis of variance test for continuous variables and the chi-square test and the Linear by linear association method for categorical variables. A P value of <0.05 was considered statistically significant.
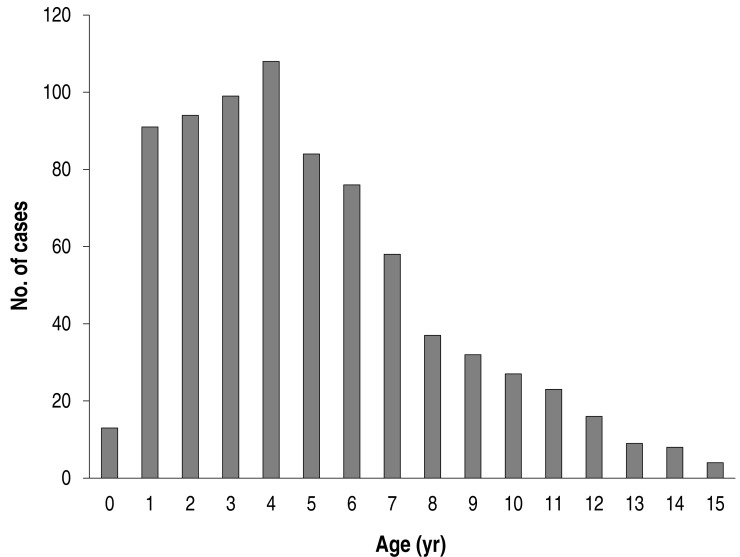
The mean age was 5.0±3.2 years of age (a range of 4 months to 15 years), and the male-to-female ratio was 1:1 (398:381). The age distribution of the patients is shown in Fig. 1. The highest number was noted at 4 years of age (108/779), and most children were distributed in 1-6 years of age (70.9%, 552/779); 87 patients were >10 years of age, accounting for 11.2% of all patients (87/779).
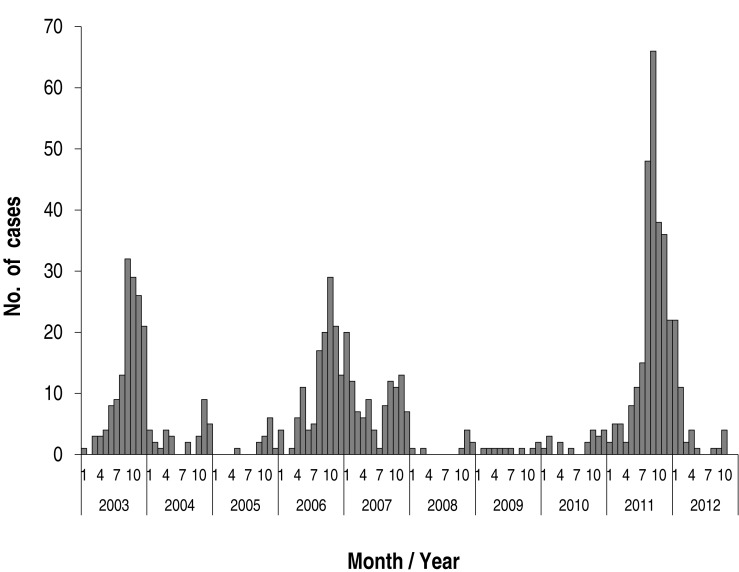
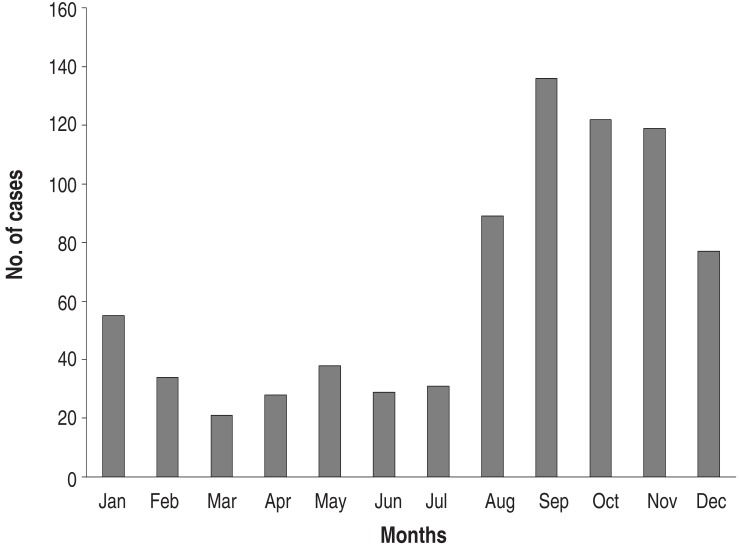
Annual cases during the study period are shown in Fig. 2. There were markedly fluctuating frequencies over the years. Epidemics were noted in 2003 (149 cases; 19.1% of total subjects), in 2006-2007 (241 cases; 30.1%, 131 cases in 2006), and 2011 (258 cases; 33.1 %). Thus, epidemics occurred at intervals of 3-4 years during the study period (Fig. 2). The monthly cases for the total study period are shown in Fig. 3. In the 2003 and 2011 epidemics, the epidemic period was within a year, but it was longer in 2006-2007. In total cases, the highest number of the patients was seen in September (136 cases, 17.5%), and nearly half of patients were seen between September and November (autumn, 377 cases, 48.4%). There were relatively fewer patients seen from February to July. In seasonal frequency, the largest number of cases was seen in the autumn, followed by winter (December to February, 166 cases, 21.3%), summer (June to August, 149 cases, 19.1%), and spring (March to May, 87 cases, 11.2%) (Fig. 4).
When we analyzed the data from the 3 epidemics, we found that the epidemiological pattern of 2003, 2006, and 2011 was relatively similar (Fig. 3). Thus, we tried to compare the epidemiological parameters among the patients who were admitted from March of the epidemic year to February of the next year, although the 2006 epidemic had a longer epidemic period. The total subjects for this comparison were 598: 155 in 2003-2004, 159 in 2006-2007, and 284 in 2011-2012, respectively. The mean age of the total patients was 5.1±3.2 years, and that of each group was 4.7 ±2.6 years, 5.5±3.1 years, and 5.3±3.7 years, respectively with no statistical difference (P=0.1). The male-to-female ratio of the total patients was 1:1 (307:291), and that of each group was 1.2:1 (85:70), 1:1.3 (70:89), and 1.2:1 (152:132), respectively, and the female-predominance was noted in the 2006 epidemic. The mean hospital stay was 6.7±2.6 days (ranged 3 to 22 days), and there was no difference across the groups. The rates of seroconversion during hospitalization were 36% of total patients (215/598); among the groups, the rates were 43% (67/155), 33.3% (53/159), and 33.5% (95/284), respectively (Table 1).
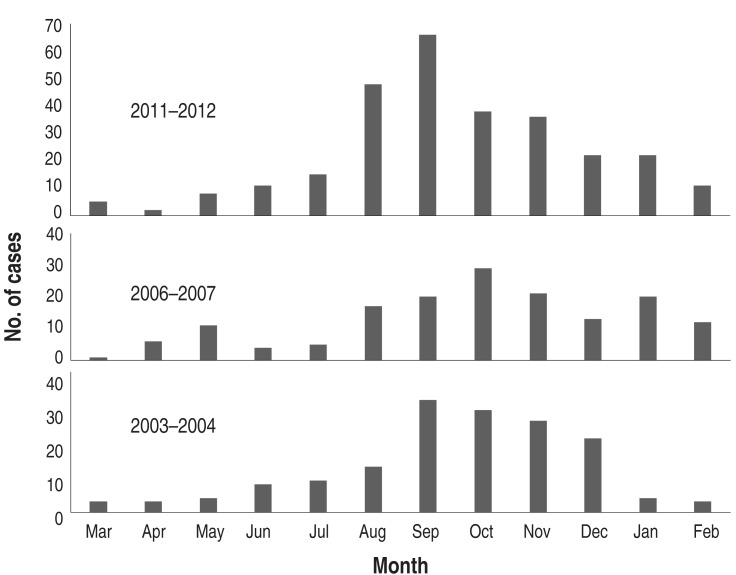
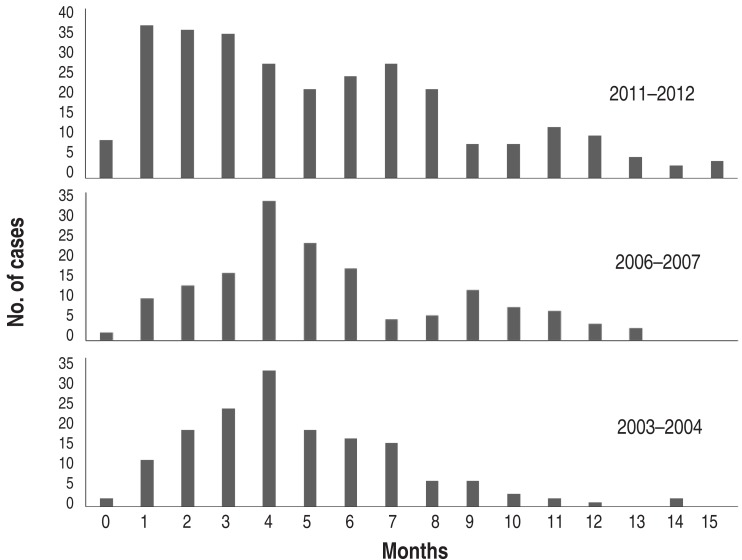
During the one-year period of each epidemic, the pattern of monthly cases was similar, but peak months were seen in September in 2003 and 2011 and October in 2006. After the peak month, the cases gradually decreased until spring (Fig. 5). The 2006 outbreak extended over a year to 2007, but a similar pattern of autumn predominance was seen in 2007 (Fig. 3). Although the mean age of children with MP pneumonia was not different among the groups, the peak age groups in children in 2003 and 2006 were the 3-6 year-olds (57.4% and 56%, respectively), but in the 2011 epidemic it was the 1-4 year-olds (46.5%). In addition, the proportion of the patients under 2 years age group was 20%, 15.1% and 28.2% of total patients, and of the >10 years of age group was 5.2%, 13.8% and 14.8% of total patients, respectively (Fig. 6).
In this study, we found that MP pneumonia epidemics in our city have occurred 3 times in a single decade, with 3- to 4-year cycles. Cyclic epidemics have been reported in other areas in Korea since the early 1980s9,10,11), and they have also been reported in other countries, including the United States, Denmark and Japan with timing variations of 3-7 years3,4,5,6,7,8). In these epidemiological studies, most epidemics lasted less than a year, but some epidemics persisted longer, as in 2006-2007 in this study. Sporadic cases continue to be observed in interepidemic years. We found that the epidemiological pattern of MP pneumonia in Korea, such as the pattern of monthly cases during an epidemic, was relatively similar across the epidemics, although we found some variation in the duration of the epidemic, age distribution, and the month of the peak cases. In each epidemic, the patients began to appear in early summer, peaked in September or October, and then slowly faded after several months or more. The monthly (seasonal) cases of the total patients (n=779) were higher from September to November (autumn), and lower from February to July. The prevalent months of other respiratory virus infections differ according to pathogen, and seasonal influenza is most prevalent from January to April in Korea [unpublished observation]. Previous epidemiological studies showed variable seasonal predominance3,4,6,7), and a 2011 epidemic in Europe indicated that patients occurred predominantly in autumn and winter12).
Historically MP pneumonia has been known to be more prevalent in older children, including adolescents1), but the most prevalent patients in Korea were 3-6 years with a trend toward younger age in recent epidemics9,10,11). In this series, 1-6 years of age was predominant (70.9%), and over 10-year-old patients were relatively rare (11.2%), although children of all ages were affected. Furthermore, the peak age distribution of the 2011 epidemic shifted toward a younger age (1-3 years of age) than those of the 2003 and 2006 epidemics (3-5 years of age) (Fig. 6). Recently, a large proportion of children younger than 5 years has also been affected in MP pneumonia epidemics in Western countries13,14,15).
The pattern of age distribution, the 3- to 4-year cyclic epidemics, and the rarity of occurrence in adults in Korea suggest that the epidemiologic characteristics of MP are similar to those of systemic viral diseases such as measles in the prevaccine era2). A nonimmune young age group would accrue during the 3- to 4-year interepidemic period, and as epidemics repeated, the susceptible groups would move to younger children; most children would thereby acquire immunity to MP (or measles) before reaching adulthood. Thus, the populations that have a longer duration of epidemic cycles may have more young adult patients in MP pneumonia epidemics. This assumption is also supported by seroprevalence studies of MP, which found IgG antibodies showing progressively greater positive rates along with increasing ages16,17).
It is believe that the genotype of MP may be changing and the variant of genomic materials might be diverse in each epidemic. It was reported that the main P1 subtype of one epidemic was changed in other epidemics18), and a recent study reported that polyclonal strains were detected in a single epidemic19). Since late 2000s, a high prevalence of macrolide-resistant MP (MRMP) strains, ranging 60%-90% in children, has been detected in Far East Asia, including Japan, China, and Korea20,21,22). A study group in Korea has reported that 63% of the isolated MP strains in the 2011 epidemic were MRMP strains22). Considering the age distribution of the three epidemics in this study, MRMP strains might also be influenced by pre-epidemic immune status of the population against MP (herd immunity). At the present time, a suitable treatment for MRMP-infected children has not been determined. Although some investigators reported the possibility of using tetracyclines or fluoroquinolones as alternative drugs for children with MRMP pneumonia23,24), MP pneumonia is a self-limiting disease and these drugs are not recommended for use in children due to complications such as teeth discoloration and potential cartilage damage25).
One major concern about epidemiological and clinical studies for MP infection is patient-selection bias. Early diagnosis of MP pneumonia is limited if a single serological test is used because of a lack of IgM antibodies in early stages (false negative) and persistent long-term IgM positivity for several months to more than a year (false positive)1,2). In this study, we used two IgM antibodies and examined two times, at presentation and around discharge, for patient selection since the 2003 epidemic26,27,28). We found that seroconversion rates were similar across the three epidemics, 30%-40%, and those patients would be missed if a single serological test was used at presentation. Furthermore, we found that patients with a prolonged duration of seroconversion seemed to have severe disease, especially older children28). There exist long-term carriers of MP during epidemics, especially those in young children29). Additionally, the polymerase chain reaction (PCR) results can be affected by the stage of the disease, the samples of different respiratory sites or other technical errors30), and the results are not corelated with serologic results because of existence of many PCR false negative patients, especially those infected with MRMP strains26,31). Therefore, a PCR study alone at presentation has limited usefulness for clinical or epidemiological studies on MP infection. Our patient-selection policy in this study could help to overcome the early diagnostic dilemma and reduce false-positive and false-negative results as much as possible in post- and interepidemic periods.
There were some limitations in this study. Not all pneumonia patients, nor all MP pneumonia patients, were examined for diagnostic antibodies twice during the study period. Therefore, MP pneumonia patients in this series might be underestimated, but most pneumonia patients in MP pneumonia epidemic periods received two antibody tests. Since we analyzed only admitted patients, our epidemiologic data might differ from the true epidemiological pattern of MP infection in Korea.
In conclusion, MP epidemics occurred every 3-4 years in our city along with nationwide epidemics. The pattern of recent three epidemics was similar in the demographic characteristics, including age, sex and age distribution, and in the monthly-case pattern (seasonality) of the epidemic with some variations in each epidemic. In Korea, the age of affected children was younger than previously reported in other populations, and in the recent 2011 epidemic, there was a trend toward even younger age.
Conflicts of interest
Conflicts of interest:
No potential conflict of interest relevant to this article was reported.
References
1. Atkinson TP, Balish MF, Waites KB. Epidemiology, clinical manifestations, pathogenesis and laboratory detection of Mycoplasma pneumoniae infections. FEMS Microbiol Rev 2008;32:956–973.



2. Lee KY. Pediatric respiratory infections by Mycoplasma pneumoniae. Expert Rev Anti Infect Ther 2008;6:509–521.


3. Foy HM, Kenny GE, Cooney MK, Allan ID. Long-term epidemiology of infections with Mycoplasma pneumoniae. J Infect Dis 1979;139:681–687.


4. Ponka A. Occurrence of serologically verified Mycoplasma pneumoniae infections in Finland and in Scandinavia in 1970-1977. Scand J Infect Dis 1980;12:27–31.


5. Lind K, Benzon MW, Jensen JS, Clyde WA Jr. A seroepidemiological study of Mycoplasma pneumoniae infections in Denmark over the 50-year period 1946-1995. Eur J Epidemiol 1997;13:581–586.


6. Rastawicki W, Kałuzewski S, Jagielski M. Occurrence of serologically verified Mycoplasma pneumoniae infections in Poland in 1970-1995. Eur J Epidemiol 1998;14:37–40.


7. Hauksdottir GS, Love A, Sigurdardottir V, Jonsson T. Outbreaks of Mycoplasma pneumoniae infections in Iceland 1987 to 1997: a ten and a half years review. Eur J Epidemiol 1999;15:95–96.


8. Ito I, Ishida T, Osawa M, Arita M, Hashimoto T, Hongo T, et al. Culturally verified Mycoplasma pneumoniae pneumonia in Japan: a long-term observation from 1979-99. Epidemiol Infect 2001;127:365–367.



9. Kang KS, Woo HO. Pattern of occurrence of Mycoplasma pneumoniae pneumonia in admitted children: Southern Central Korea, from 1989 to 2002. J Korean Pediatr Soc 2003;46:474–479.
10. Kim JW, Seo HK, Yoo EG, Park SJ, Yoon SH, Jung HY, et al. Mycoplasma pneumoniae pneumonia in Korean children, from 1979 to 2006: a meta-analysis. Korean J Pediatr 2009;52:315–323.

11. Eun BW, Kim NH, Choi EH, Lee HJ. Mycoplasma pneumoniae in Korean children: the epidemiology of pneumonia over an 18-year period. J Infect 2008;56:326–331.


12. Lenglet A, Herrador Z, Magiorakos AP, Leitmeyer K, Coulombier D. European Working Group on Mycoplasma pneumoniae surveillance. Surveillance sta tus and recent data for Mycoplasma pneumoniae infections in the European Union and European Economic Area, January 2012 2012;17:pii=20075
13. Othman N, Isaacs D, Kesson A. Mycoplasma pneumoniae infections in Australian children. J Paediatr Child Health 2005;41:671–676.


14. Defilippi A, Silvestri M, Tacchella A, Giacchino R, Melioli G, Di Marco E, et al. Epidemiology and clinical features of Mycoplasma pneumoniae infection in children. Respir Med 2008;102:1762–1768.


15. Gadsby NJ, Reynolds AJ, McMenamin J, Gunson RN, McDonagh S, Molyneaux PJ, et al. Increased reports of Mycoplasma pneumoniae from laboratories in Scotland in 2010 and 2011 - impact of the epidemic in infants. Euro Surveill 2012;17:pii=20110
16. Brunner H, Prescott B, Greenberg H, James WD, Horswood RL, Chanock RM. Unexpectedly high frequency of antibody to Mycoplasma pneumoniae in human sera as measured by sensitive techniques. J Infect Dis 1977;135:524–530.


17. Nir-Paz R, Michael-Gayego A, Ron M, Block C. Evaluation of eight commercial tests for Mycoplasma pneumoniae antibodies in the absence of acute infection. Clin Microbiol Infect 2006;12:685–688.


18. Kenri T, Okazaki N, Yamazaki T, Narita M, Izumikawa K, Matsuoka M, et al. Genotyping analysis of Mycoplasma pneumoniae clinical strains in Japan between 1995 and 2005: type shift phenomenon of M. pneumoniae clinical strains. J Med Microbiol 2008;57(Pt 4): 469–475.


19. Pereyre S, Charron A, Hidalgo-Grass C, Touati A, Moses AE, Nir-Paz R, et al. The spread of Mycoplasma pneumoniae is polyclonal in both an endemic setting in France and in an epidemic setting in Israel. PLoS One 2012;7:e38585



20. Morozumi M, Takahashi T, Ubukata K. Macrolide-resistant Mycoplasma pneumoniae: characteristics of isolates and clinical aspects of community-acquired pneumonia. J Infect Chemother 2010;16:78–86.


21. Cao B, Zhao CJ, Yin YD, Zhao F, Song SF, Bai L, et al. High prevalence of macrolide resistance in Mycoplasma pneumoniae isolates from adult and adolescent patients with respiratory tract infection in China. Clin Infect Dis 2010;51:189–194.


22. Hong KB, Choi EH, Lee HJ, Lee SY, Cho EY, Choi JH, et al. Macrolide resistance of Mycoplasma pneumoniae, South Korea, 2000-2011. Emerg Infect Dis 2013;19:1281–1284.



23. Okada T, Morozumi M, Tajima T, Hasegawa M, Sakata H, Ohnari S, et al. Rapid effectiveness of minocycline or doxycycline against macrolide-resistant Mycoplasma pneumoniae infection in a 2011 outbreak among Japanese children. Clin Infect Dis 2012;55:1642–1649.


24. Kawai Y, Miyashita N, Kubo M, Akaike H, Kato A, Nishizawa Y, et al. Therapeutic efficacy of macrolides, minocycline, and tosufloxacin against macrolide-resistant Mycoplasma pneumoniae pneumonia in pediatric patients. Antimicrob Agents Chemother 2013;57:2252–2258.



25. Principi N, Esposito S. Macrolide-resistant Mycoplasma pneumoniae: its role in respiratory infection. J Antimicrob Chemother 2013;68:506–511.


27. Lee KY, Lee HS, Hong JH, Lee MH, Lee JS, Burgner D, et al. Role of prednisolone treatment in severe Mycoplasma pneumoniae pneumonia in children. Pediatr Pulmonol 2006;41:263–268.


28. Youn YS, Lee KY, Hwang JY, Rhim JW, Kang JH, Lee JS, et al. Difference of clinical features in childhood Mycoplasma pneumoniae pneumonia. BMC Pediatr 2010;10:48



29. Dorigo-Zetsma JW, Wilbrink B, van der Nat H, Bartelds AI, Heijnen ML, Dankert J. Results of molecular detection of Mycoplasma pneumoniae among patients with acute respiratory infection and in their household contacts reveals children as human reservoirs. J Infect Dis 2001;183:675–678.


Fig. 5
Monthly cases of Mycoplasma pneumoniae pneumonia in the epidemic years 2003-2004, 2006-2007, and 2011-2012.




 PDF Links
PDF Links PubReader
PubReader PubMed
PubMed Download Citation
Download Citation


