Article Contents
| Korean J Pediatr > Volume 58(9); 2015 |
|
Abstract
Purpose
The purpose of this study was to evaluate the efficacy and safety of Montelukast sodium in the prevention of bronchopulmonarydysplasia (BPD).
Methods
The Interventional study was designed as a multicenter, prospective, and randomized trial, with open labeled and parallel-experimental groups, 66 infants were enrolled and allocated to either the case group (n=30) or the control group (n=36) based on gestational age (GA). Infants in the case group were given Montelukast sodium (Singulair) based on their body weight (BW). Zero week was defined as the start time of the study.
Results
The incidence of moderate to severe BPD was not different between the groups (case group: 13 of 30 [43.3%] vs. control group: 19 of 36 [52.8%], P=0.912). Additionally, secondary outcomes such as ventilation index, mean airway pressure and resort to systemic steroids were not significantly different. There were no serious adverse drug reactions in either group, and furthermore the rate of occurrence of mild drug related-events were not significantly different (case group: 10 of 42 [23.8%] vs. control group: 6 of 48 (15.8%), P=0.414).
Recent advances in medical treatments of bronchopulmonary dysplasia (BPD), such as the use of antenatal steroids, surfactant therapy, and mechanical ventilation have increased survival rates in preterm infants; however BPD remains, a major complication of preterm infants1,2). The incidence of BPD at 36 weeks postmenstrual age (PMA) is reported as 16%-39% in preterm infants born at less than 32 weeks gestation, or in infants with very low birth weight3,4,5). Infants born at less than 28 weeks gestation had an incidence of 40%-52% BPD at 36 weeks PMA6,7). Thus, BPD clearly remains a significant problem in preterm infants.
In 1975, Philip8) proposed that the etiology of BPD was multifactorial, mostly composed of external factors, such as exposure to oxygen and pressure. Inflammatory responses were subsequently introduced in the etiological paradigm, including external sources (chorioamnionitis, postnatal infections), iatrogenic sources (ventilation, oxygen), and the internal host response9). Even though this paradigm is confirmed by experimental data, few innovative therapies have proven efficacious10). Vitamin A, caffeine, postnatal corticosteroids, and stem cells from cord blood may help in repairing the preterm lung. Specific anti-inflammatory treatments hold some promise, but developing drugs for use in infants is a challenging task11,12).
Leukotrienes, metabolites of arachidonic acid, have potent chemotactic activity towards polymorph nuclear leukocytes. They play an important role in chronic pulmonary inflammation13), and are associated with hyperoxia and inhibition of alveolarization14). In accordance with this role, several studies on leukotrienes in BPD have been conducted recently. Sheikh et al.15) and Joung et al.16) reported that infants with clinical BPD have higher levels of urinary leukotriene E4.
Montelukast sodium is a selective cysteinyl leukotriene receptor antagonist with proven clinical benefit in the treatment of asthma and perennial allergic rhinitis, in adults and children as young as 6 months of age. Its efficacy and safety in children younger than 6 months is not yet established. Knorr et al.17) and Kearns et al.18), reported in their pharmacokinetics and safety study of Montelukast in children 1 to 3 and 3 to 6 months of age, that administration of a single dose of Montelukast (4 mg) was generally well tolerated.
The effect of Montelukast on BPD in neonatal intensive care setting have been published19), but it had small population and retrospective design. Therefore we performed this study in order to determine the efficacy and safety of Montelukast on BPD in preterm infants, with prospective, randomized trial.
This study was a multicenter, prospective, randomized, open labeled, parallel group, intervention trial. All protocols were approved by the Institutional Review Board at each site, and the studies were conducted in compliance with the Korea Food and Drug Administration. This study was registered in clinical trials database (clinicaltrials.gov, ID: NCT01717625). A parent or guardian provided written informed consent for every infant included in the study. We selected the participant number based on earlier clinical trials by Ambalavanan et al.20) with vitamin A (where superiority limit was set to 10%); as well as a study on BPD by Dani et al.21) with incidence confirmed at 40%. They reported the combination of death and BPD (BPD/death) between inhaled nitric oxide and control groups, and the proportion of the BPD/death of control group was 90%. Based on these data, we assumed that the difference of morbidity and mortality of BPD between two groups is 40%, and the rate of morbidity and mortality of BPD is 90%. Accordingly, statistical power 80%, type I error 0.025 was set. In addition, we included 72 patients based on a previous study with 60 patients with a 20% exclusion rate.
Inclusion criteria were preterm infants born at less than 32 weeks gestational age (GA); infants 14 days after birth on oxygen or mechanical ventilation; infants with more than 20 calories per kilogram per day by enteral feeding; All inclusion and participation in clinical trials was by signed parental consent. Exclusion criteria were any of the following: infants with congenital anomaly; infants from who blood sampling for pharmacokinetics was impossible due to cardiovascular collapse; in addition, any cases in which clinical trials were deemed difficult by the investigator. Examinees confirmed by inclusion criteria, were registered in the trial and allocated to case or control groups (1:1) by random assignment, stratified by GA (>28 weeks, ≤28 weeks), using shuffled blocks of random numbers in Microsoft Office, Excel 2007. Infants assigned to the test group, were given Montelukast sodium according to body weight (less than 1,000 g, 0.5 mg; 1,000 g to 1,500 g, 1.0 mg; 1,500 g to 2,000 g, 1.5 mg; greater than 2,000 g, 2 mg). The drug was given once daily via orogastrictube or by oral administration, at a given time. Each infant was treated until 36 weeks GA or until discharge. The body weight was measured every week to determine accuracy of dosage. All enrolled patients received surfactant treatment.
All clinical assessments and data collections were performed prospectively by local investigators, who were all trained pediatricians. The evaluation of data included: (1) efficacy of the drug, (2) safety of the drug, and (3) pharmacokinetic evaluation. Zero week was defined as the start time of the study.
The efficacy of the drug was evaluated by incidence and severity of BPD (primary outcome)22). In secondary outcome, we evaluated ventilation index [VI=R×(PIP-PEEP)×PaCO2/1,000], mean airway pressure {MAP=[R×It×PIP+(60-R×It×PEEP)]/60} (R, rate; PIP, peak inspiratory pressure; PEEP, positive end expiratory pressure; It, inspiratory time). We also evaluated usage of mechanical ventilation, oxygen, systemic steroid and change in body weight. Systemic steroid was used only for rescue therapy of BPD. The principles of ventilator therapy was reflected by recent ventilator trend (early extubation and nasal continuous positive airway pressure after surfactant treatment), through the meeting.
The safety of the drug was evaluated by the rate of adverse events and classified by System Organ Classes (SOC) classification. Each adverse event was also classified by intensity, progress, complication, and treatment-causal relationship.
Seven of 36 Montelukast groups who agreed to inclusion in pharmacokinetic studies, were divided into A and B groups by using shuffled blocks of random numbers. In each center, subjects were also further divided according to sampling time, as single dose study groups as follows; A group: at 2, 6 hours after medication, B group: at 4, 24 hours after medication; and as multiple dose study group as follows: A group: at 2,6 hours on the 7th day post medication, B group: at 4,24 hours on the 7th day post medication. Pharmacokinetic samples were analyzed using BioInfra (Seoul, Korea). Quantification of Montelukast was done by ultra performance liquid chromatography-mass spectrometry (MS)/MS. The results were calculated as follows: C=1/x2 by linear regression using MassLynx V4.1 (Waters Co., Milford, MA, USA) {C=concentration, x=[Montelukast peak area/Internal standards (ISTD) peak area]×ISTD concentration}. Each preterm infant was evaluated for ventilating mode (mode, fiO2, MAP, oxygen index), medication history, weight, associated disease (intraventricular hemorrhage [IVH], hemodynamically significant patent ductus arteriosus, necrotizing entrocolitis [NEC], pneumonia, sepsis), laboratory data (hemoglobin [Hb], hematocrit, platelet count, blood urea nitrogen, creatinine, aspartate transaminase, alanine transaminase, and blood culture). Proinflamatory cytokines were measured in intubated infants only at the time of enrollment, and weekly upto 2 weeks after taking medicine. We set the end point of the studies as 36 weeks GA, or the discharge date.
The results were presented as mean and standard deviation for continuous variables, and frequency and percentage for categorical variables. The superiority test with R 2.14.1 (R Foundation for Statistical Computing, Vienna, Austria) was used for primary outcome. The secondary efficacy rating variables between two groups are done by t test and Fisher exact test. The t test was used for ventilation index and mean airway pressure. Fisher exact test was used for demographic data, usage of mechanical ventilation and steroids.
The safety of the drug evaluation was performed by Fisher exact test comparing the incidence and severity of adverse reaction, as well as causality of adverse with drug administration, between two groups. Vital signs, physical examinations, and laboratory results are evaluated by descriptive statistic comparisons between two groups.
Pharmacokinetic modeling was done with a single compartmental model. Because of insufficient information about the absorption period, the modeling of intravenous administration which excludes absorption modeling, was assumed. Based on this, covariate analyses like age, weight, and sex were added. The evaluation of the developed model was done by three methods: (1) relative standard error (standard error/estimate value) where sensitivity of parameter estimate value less than 50% is reliable, (2) visual inspection 1: comparison of similarity between longitudinal progress of the predicted value and observed value, (3) visual inspection 2: in the group of individual, evaluate bias whether weighted residual is distributed around a line of zero (weighted residual=residual/observed value).
A total of 83 infants enrolled in 5 units, but only 77 infants constituted the study group; 1 infant was excluded due to lack of parental consent, 1 infant based on exclusion criteria, 1 infant due to excess number, and 3 infants for violation of medication protocol. Among the 77 infants, 37 enrolled in the case group and 40 in the control group. 7 infants of the case group were terminated early; 3 infants were excluded for onset of comorbidity; 1 infant for treatment with phenobarbital; 1 infant for the lack of parental consent; 1 infant for protocol violation; 1 infant based on the researcher's opinion. Four infants of the control group were terminated early; 2 infants for medication of Montelukast; and 2 infants for protocol violation (Fig. 1). The characteristics of the patients in the 2 groups are shown (Table 1). There was no difference in birth weight (case group: 1,097±327.3 g vs. control group: 997±235.3 g, P=0.153) and GA (case group: 27.6 ±1.4 weeks vs. control group: 27.3±1.6 weeks, P=0.374) between the two groups, at birth. Additionally, there were no significant differences in other characteristics (Apgar scores, usage of antenatal steroid, the incidence once, and IVH).
The incidence of moderate to severe BPD was not different between the groups. (case group: 43.3% vs. control group: 52.8%, P=0.912) (primary outcome, Table 2). There were no significant differences in FiO2 at 2 weeks after treatment (case group: 0.28% ±0.07% vs. control group: 0.29%±0.08%, P=0.472); MAP (case group: 6.33±2.25 mmHg vs. control group: 8.63±1.92 mmHg, P= 0.062); ventilation index (case group: 23.1±13.8 vs. control group: 18.5±9.6, P=0.507); need of invasive ventilator (case group: 7 of 30 [23.30%] vs. control group: 7 of 36 [19.40%], P=0.131), use of systemic steroids for rescue therapy of BPD (case group: 7 of 30 [23.3%] vs. control group: 7 of 36 [19.4%], P=0.768) (secondary outcome, Table 3).
The rate of adverse event did not differ between the groups (case group: 10 of 42 [23.8%] vs. control group: 6 of 32 [15.8%], P=0.414). There were no serious adverse drug events. According to SOC classification, the most common adverse event is infection (case group: 8 vs. control group 3, total 11). These included staphylococcal bacteremia, other sepsis, candida infection, and septic shock. Next are gastrointestinal disorders (abdominal distension, NEC, ileus); abnormal serum chemistry (elevation of liver enzyme); and blood and lymphatic system disorders(thrombocytopenia, anemia) (Table 4). Intensity of adverse events was more severe in case group (case group: 11 vs. control group: 0, P=0.023). However, most of the adverse reactions were evaluated as unlikely to be causally related to Montelukast treatment (data not shown). The laboratory findings demonstrated that the number of clinically significant (CS) abnormal platelet count was increased significantly in the case group (case group: 6 vs. control group: 1, P=0.043), while the number of nonclinically significant (NCS) abnormal platelet count was increased in the control group rather than case group. There were no significant differences between groups in the other laboratory findings (Table 5). Importantly, there were no significant differences in CS rate [CS/(normal +NCS)] of Hb (case group: 0.01 vs. control group: 0.00, P=0.621), platelet count (case group: 0.02 vs. control group: 0.00, P=0.066), blood culture (case group: 0.05 vs. control group: 0.03, P=0.489) and other laboratory findings (data not shown).
Seven infants from 3 units enrolled to the pharmacokinetic study. Among 17 infants, 9 enrolled in the single dose group and 8 in the multiple dose group. Pharmacokinetic modeling using the modeling of intravenous administration (excluding absorption) and covariate analysis like age, weight, and sex resulted in the following:
TVV = θ1· (CR/CRmed)θ2
V = TVV · EXP (η)
TVCL = θ3 · (AGE/AGEmed)θ4
CL = TVCL
Y = PRED · (1+ɛ)
TVV is a typical value of volume of distribution, TVCL is a typical value of clearance, volume (V) and clearance (CL) are each individual value, CRmed and AGEmed are median value of the level of serum creatinine (CR) and the age of infant (AGE) (CRmed=0.6 mg/dL, AGEmed=27.0 days). η is a random variable of inter-individual difference, but clearance is not significant, a typical value of group reveals individual value. Y is an observed value, PRED is a predicted value, ɛ is a random variable related residual error or intraindividual difference.
The concentration of Montelukast according to the time is shown (Fig. 2), the characteristics of the patients in the 2 groups were not significantly different. As shown in Table 6, each typical value of distribution volume and clearance is 918 mL and 5.12 mL/hr. According to covariate analysis, the volume of distribution decreases as the serum creatinine concentration increases, and the clearance decreases as the age increases (P<0.0005). The relative standard error of estimated parameters is 7.2% to 34.6%, less than 50%, thus the modeling results are reliable (Table 6). In the 1mg group with the highest number of participants, the predicted value (line) passes close to the center of observed values (dots), therefore, we concluded that the modeling was appropriate (Fig. 3).
BPD originally described by Northway in 1967, comprised the clinical, radiologic and pathologic changes seen in infants with severe respiratory distress syndrome (RDS) or prolonged mechanical ventilation and high inspiratory oxygen levels23). The lung pathology included inflammation, airway fibrosis and smooth muscle hypertrophy, alveolar collapse and hyperinflation and interstitial fibrosis of all tissues24). The "new BPD" cases are more compatible with an arrest in lung development than with mechanical injury. Inflammation was introduced in this paradigm, thus including external sources (chorioamnionitis, postnatal infections), iatrogenic sources (ventilation, oxygen), and the internal host response9). Despite confirmation of this paradigm by experimental data, few innovative therapies have proven efficacious10). Vitamin A, caffeine, postnatal corticosteroids, and stem cells from cord blood may help repair the preterm lung. Kim et al.19), reported Montelukast as an adjunct therapy, in BPD. Montelukast (1 mg/kg/day) was administered to 15 preterm infants with established BPD for an average period of 12 weeks. Ventilation index was significantly improved after 2 weeks in the Montelukast treatment group, and there were no differences in the incidence of adverse reaction between the 2 groups. While specific anti-inflammatory treatments hold some promise, developing drugs for infants is a difficult task11,12).
In our study, there were no differences in predictors of efficacy, such as incidence and severity of BPD, FiO2, MAP, ventilation index, the need of ventilator, or the use of systemic steroid at 4 weeks after taking medicine. And similar to previous studies that reported in pharmacokinetics and safety study of Montelukast in children 1 to 3 and 3 to 6 months of age17,18), administration of Montelukast was well tolerated.
This multicenter, prospective, and randomised study, has limitations. First, the protective effects of an effective drug must involve the inhibition of leukotriene production and not be attributed solely to anti-inflammatory mechanisms25). Leukotrienes are known mediators of hyperoxia-induced aberrant alveolarization14,26,27). It has been suggested that apart from inflammatory processes, the mechanisms involved in Leukotrienes mediated aberration of alveolarizationmay require subtle alterations in the signal transduction pathways of growth factors and receptors involved in the alveolarization process. Based on these mechanisms, instead of leukotriene receptor antagonist, other alternates under consideration are the leukotriene synthesis inhibitors, like 5-lipoxygenase inhibitor (e.g., Zileuton) or 5-lipoxygenase-activating protein inhibitor (e.g., MK-0591, not approved by Food and Drug Administration). Second, given the multifactorial etiology of BPD, these finding cannot be generalized. Charafeddine et al.28) subclassified BPD into two groups according to the criteria; 'classic' and 'atypical' BPD. 'Atypical BPD' was diagnosed in BPD cases without RDS or in BPD cases that were preceded by initial RDS that resolved within 10 days, and required no oxygen supplementation for at least 72 hours, beyond the 28-day oxygen requirement. Joung et al.16) reported that although there was no significant difference between the no/mild and the moderate/severe BPD groups, when they compared 'classic' and 'atypical' BPD groups, there was a significant increase in urinary LTE4 levels on day 7 in the 'atypical' BPD group. This observation has two interpretations. The evaluation of the incidence and severity of all BPD can mask that of 'atypical BPD', and secondly, the evaluation of the total treatment period can mask of the effects of the early period related to inflammatory insult. Third, the differences (40%) shown in previous studies21) might be exaggerated due to smaller sample size. If the sample size is increased by the participation of more units, the differences can decrease. Forth, the effectiveness of the orally given drug was influenced by variable volume of enteral feeds, they could influence drug availability and absorption from the gastrointestinal tract. Fifth, considering the multicenter study, we do not standardize the protocol exactly for each center. (e.g., dosage of systemic steroid, protocol of ventilator therapy)
Several pediatric studies with Montelukast show that oral Montelukast is generally well tolerated29,30,31). Bisgaard et al.32) reported the safety and tolerability of Montelukast in a placebo-controlled and open-labeled trial. The most common clinical adverse events they reported were upper respiratory infection, worsening pulmonologic problem, and fever. And there were no clinically meaningful differences in laboratory parameters. In addition, Sarkar et al.33) reported infant outcomes in a prospective study with Montelukast, during pregnancy. According to this research group, Montelukast does not appear to increase the basal rate of major malformations, moreover, the resultant lower birth weight was most likely associated with the severity of the maternal condition, rather than the treatment In our study, there was no serious adverse drug event, and known adverse event (described above) did not differ between the groups.
In conclusion, Montelukast was not effective in reducing the moderate or severe BPD. Additionally, there was no significant increase in adverse drug event associated with Montelukast treatment. Nevetheless, this study is significant in that it is a first prospective study of Montelukast for preterm infant. Based on designing and finding from the present study, it is expected that further studies are needed on the relationship between BPD and leukotriene modifiers.
Acknowledgment
This work was supported by the research fund of the Korea Food and Drug Administration (KFDA).
Conflicts of interest
Conflicts of interest:
No potential conflict of interest relevant to this article was reported.
References
1. Baveja R, Christou H. Pharmacological strategies in the prevention and management of bronchopulmonary dysplasia. Semin Perinatol 2006;30:209–218.


3. Zeitlin J, Draper ES, Kollee L, Milligan D, Boerch K, Agostino R, et al. Differences in rates and short-term outcome of live births before 32 weeks of gestation in Europe in 2003: results from the MOSAIC cohort. Pediatrics 2008;121:e936–e944.


4. Choi CW, Kim BI, Kim EK, Song ES, Lee JJ. Incidence of bronchopulmonary dysplasia in Korea. J Korean Med Sci 2012;27:914–921.



5. Payne NR, LaCorte M, Karna P, Chen S, Finkelstein M, Goldsmith JP, et al. Reduction of bronchopulmonary dysplasia after participation in the Breathsavers Group of the Vermont Oxford Network Neonatal Intensive Care Quality Improvement Collaborative. Pediatrics 2006;118(Suppl 2): S73–S77.


6. Laughon M, Allred EN, Bose C, O'Shea TM, Van Marter LJ, Ehrenkranz RA, et al. Patterns of respiratory disease during the first 2 postnatal weeks in extremely premature infants. Pediatrics 2009;123:1124–1131.



7. Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC, et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics 2010;126:443–456.



8. Philip AG. Oxygen plus pressure plus time: the etiology of bronchopulmonary dysplasia. Pediatrics 1975;55:44–50.



9. Speer CP. New insights into the pathogenesis of pulmonary inflammation in preterm infants. Biol Neonate 2001;79:205–209.


10. Wright CJ, Kirpalani H. Targeting inflammation to prevent bronchopulmonary dysplasia: can new insights be translated into therapies? Pediatrics 2011;128:111–126.



12. Martin RJ, Fanaroff AA. The preterm lung and airway: past, present, and future. Pediatr Neonatol 2013;54:228–234.


13. Beller TC, Friend DS, Maekawa A, Lam BK, Austen KF, Kanaoka Y. Cysteinyl leukotriene 1 receptor controls the severity of chronic pulmonary inflammation and fibrosis. Proc Natl Acad Sci U S A 2004;101:3047–3052.



14. Phillips GJ, Mohammed W, Kelly FJ. Oxygen-induced lung injury in the pre-term guinea pig: the role of leukotriene B4. Respir Med 1995;89:607–613.


15. Sheikh S, Null D, Gentile D, Bimle C, Skoner D, McCoy K, et al. Urinary leukotriene E(4) excretion during the first month of life and subsequent bronchopulmonary dysplasia in premature infants. Chest 2001;119:1749–1754.


16. Joung KE, Kim HS, Lee J, Shim GH, Choi CW, Kim EK, et al. Correlation of urinary inflammatory and oxidative stress markers in very low birth weight infants with subsequent development of bronchopulmonary dysplasia. Free Radic Res 2011;45:1024–1032.


17. Knorr B, Maganti L, Ramakrishnan R, Tozzi CA, Migoya E, Kearns G. Pharmacokinetics and safety of montelukast in children aged 3 to 6 months. J Clin Pharmacol 2006;46:620–627.


18. Kearns GL, Lu S, Maganti L, Li XS, Migoya E, Ahmed T, et al. Pharmacokinetics and safety of montelukast oral granules in children 1 to 3 months of age with bronchiolitis. J Clin Pharmacol 2008;48:502–511.


19. Kim HM, Song JE, Lee SM, Park MS, Park KI, Namgung R, et al. Montelukast as an add-on therapy in bronchopulmonary dysplasia. Korean J Pediatr 2009;52:181–186.

20. Ambalavanan N, Wu TJ, Tyson JE, Kennedy KA, Roane C, Carlo WA. A comparison of three vitamin A dosing regimens in extremely-low-birth-weight infants. J Pediatr 2003;142:656–661.


21. Dani C, Bertini G, Pezzati M, Filippi L, Cecchi A, Rubaltelli FF. Inhaled nitric oxide in very preterm infants with severe respiratory distress syndrome. Acta Paediatr 2006;95:1116–1123.


23. Northway WH Jr, Rosan RC, Porter DY. Pulmonary disease following respirator therapy of hyaline-membrane disease. Bronchopulmonary dysplasia. N Engl J Med 1967;276:357–368.


24. Bonikos DS, Bensch KG, Northway WH Jr, Edwards DK. Bronchopulmonary dysplasia: the pulmonary pathologic sequel of necrotizing bronchiolitis and pulmonary fibrosis. Hum Pathol 1976;7:643–666.


25. Park MS, Sohn MH, Kim KE, Park MS, Namgung R, Lee C. 5-Lipoxygenase-activating protein (FLAP) inhibitor MK-0591 prevents aberrant alveolarization in newborn mice exposed to 85% oxygen in a dose- and time-dependent manner. Lung 2011;189:43–50.


26. Manji JS, O'Kelly CJ, Leung WI, Olson DM. Timing of hyperoxic exposure during alveolarization influences damage mediated byleukotrienes. Am J Physiol Lung Cell Mol Physiol 2001;281:L799–L806.


27. Rogers LK, Tipple TE, Nelin LD, Welty SE. Differential responses in the lungs of newborn mouse pups exposed to 85% or >95% oxygen. Pediatr Res 2009;65:33–38.



28. Charafeddine L, D'Angio CT, Phelps DL. Atypical chronic lung disease patterns in neonates. Pediatrics 1999;103(4 Pt 1): 759–765.


29. Bjermer L. Montelukast in the treatment of asthma as a systemic disease. Expert Rev Clin Immunol 2005;1:325–336.


30. Nayak A, Langdon RB. Montelukast in the treatment of allergic rhinitis: an evidence-based review. Drugs 2007;67:887–901.


31. Storms W, Michele TM, Knorr B, Noonan G, Shapiro G, Zhang J, et al. Clinical safety and tolerability of montelukast, a leukotriene receptor antagonist, in controlled clinical trials in patients aged > or = 6 years. Clin Exp Allergy 2001;31:77–87.


Fig. 1
Participant flow. We selected the participant number based on earlier clinical trials by Ambalavanan et al.20) with vitamin A (where superiority limit was set to 10%); as well as a study on bronchopulmonarydysplasia (BPD) by Dani et al.21) with incidence confirmed at 40%. They reported the combination of death and BPD (BPD/death) between NO and control groups, and the proportion of the BPD/death of control group was 90%. Based on these data, we assumed that the difference of morbidity and mortality of BPD between two groups is 40%, and the rate of morbidity and mortality of BPD is 90%. Accordingly, statistical power 80%, type I error 0.025 was set. In addition, we included 72 patients based on a previous study with 60 patients with a 20% exclusion rate.
Fig. 2
Concentration of Montelukast over time. Seventeen infants in 3 NICU, enrolled in the pharmacokinetic study. They were also divided at each center, according to sampling time. 9 enrolled in the single dose study groups (A group: at 2, 6 hours after medication; B group: at 4, 24 hours after medication) and 8 enrolled in the multiple dose study group (A group: at 2,6 hours on the 7th day post medication; B group: at 4,24 hours on the 7th day post medication). The concentration of Montelukast at different time points is shown.

Fig. 3
Predicted (line) and observed values (dots) over time. The 1-mg group with the highest number of participants, predicted value (line) passes close to the center of observed values (dots), therefore, indicative of appropriate modeling. CONC, concentration; PRED, predicted value; Median, median value.
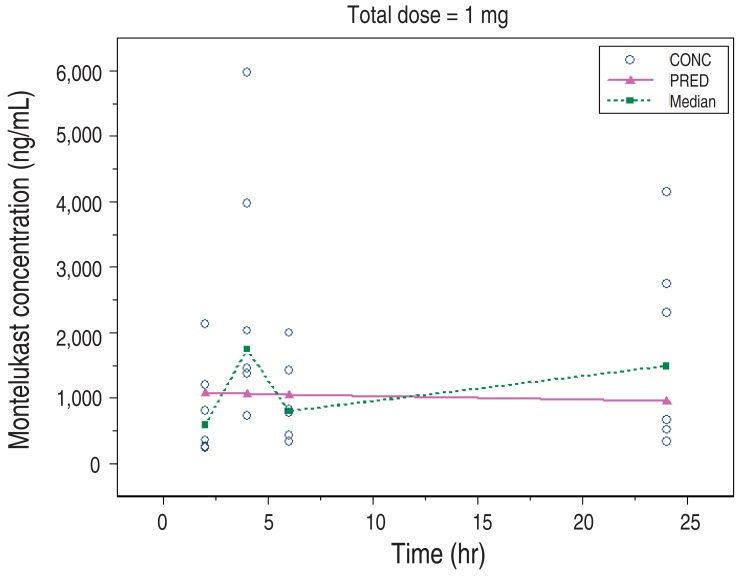
Table 1
Comparisons of demographic data of studygroups
Table 2
Incidence and severity of bronchopulmonarydysplasia (BPD)
| BPD | Case group (n=30) | Control group (n=36) | P value |
|---|---|---|---|
| Mild | 17 (56.7) | 17 (47.2) | 0.912 |
| Moderate/severe | 13 (43.3) | 19 (52.8) |



 PDF Links
PDF Links PubReader
PubReader PubMed
PubMed Download Citation
Download Citation


