Article Contents
| Korean J Pediatr > Volume 59(2); 2016 |
|
Abstract
Survival rates of preterm infants have improved in the past few decades, and central venous catheters play an important role in the intensive medical treatment of these neonates. Unfortunately, these indwelling catheters increase the risk of intracardiac thrombosis, and they provide a nidus for microorganisms during the course of septicemia. Herein, we report a case of persistent bacteremia due to methicillin-resistant Staphylococcus aureus in an extremely low birth weight (ELBW) infant, along with vegetation observed on an echocardiogram, the findings which are compatible with a diagnosis of endocarditis. The endocarditis was successfully treated with antibiotic therapy, and the patient recovered without major complications. We suggest a surveillance echocardiogram for ELBW infants within a few days of birth, with regular follow-up studies when clinical signs of sepsis are observed.
Cases of neonatal endocarditis are extremely rare, and many of the cases are reported to be unresponsive to medical treatment1,2). However, the increasing survival rate of preterm infants and use of indwelling central catheter is increasing the risk of endocarditis in neonates1,3,4). Previously, there has been a single report of an extremely low birth weight (ELBW) infant who survived methicillin-resistant Staphylococcus aureus (MRSA) endocarditis2). We report another such case of an ELBW infant with MRSA endocarditis discovered on the 9th day of life, which was successfully treated without complications.
A 960-g male neonate was born at 26 weeks and 5 days of gestation with Apgar scores of 6 and 7 at 1 and 5 minutes, respectively. He was treated with prophylactic endotracheal surfactant in the operation room just after birth, and the chest x-ray revealed a mild degree of respiratory distress syndrome. An umbilical venous catheter was inserted for the parenteral nutrition with the catheter tip in the right atrium (Fig. 1). There was no cardiac murmur, but a surveillance echocardiogram on the 4th day of age revealed a 1.2-mm patent ductus arteriosus (PDA).
On 5th day of life, blood gas analysis was consistent with metabolic acidosis, and serum total bilirubin level increased up to 10.5 mg/dL in spite of intensive phototherapy. The neonate continued to experience desaturation with bradycardia despite optimal mechanical ventilation support. After blood cultures were obtained, intravenous ampicillin-sulbactam was initiated for suspicion of sepsis. A repeat echocardiogram on the same day revealed the PDA diameter had decreased to 1 mm.
On the next day (day 6 of life), the preliminary blood culture was suspicious of gram-positive cocci, and the antibiotic regimen was changed into vancomycin. The umbilical venous catheter was removed. Hyperglycemia accompanied the persistent metabolic acidosis. The neonate became progressively hypotensive, and required continuous dopamine infusion to maintain adequate blood pressure. The tracheal aspirates showed blood-tinged sputum, and blood test results were compatible with disseminated intravascular coagulation.
On the 7th day of life, B-type natriuretic peptide (BNP) was elevated to 1,510 pg/mL, and the cardiac murmur became audible on examination. Intravenous ibuprofen was considered for treatment of PDA, but was not administered because the platelet count was 22,000/mm3. Endotracheal bleeding worsened, but cranial ultrasonography did not find any evidence of brain hemorrhage. White blood cell count was 42,300/mm3, and C-reactive protein level was 4 mg/dL.
On the 9th day of age, BNP decreased to 464 pg/mL, but the PDA diameter had increased to 1.8 mm on the third echocardiogram. Chest x-ray showed increased pulmonary congestion, and the neonate continued to require dopamine infusion. Because the hemodynamic instability appeared to be more significant than the potential risk of bleeding, intravenous ibuprofen was initiated after a transfusion of platelets.
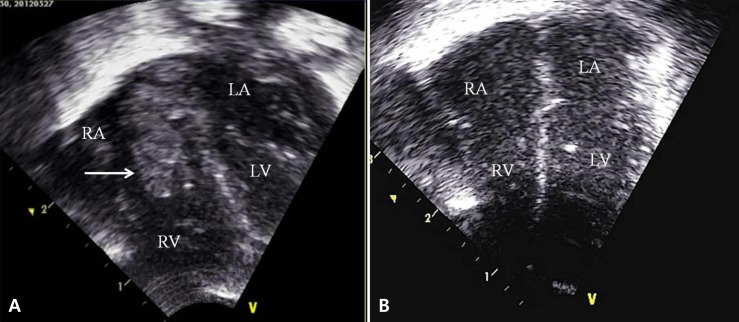
The echocardiogram done on the day 9 of life also showed a new 0.25-cm2 oscillating mass in the right atrium (Fig. 2A). The mass faced the atrial septum and the tricuspid valve – a finding compatible with vegetation of infective endocarditis. By this time, MRSA had been cultured from the blood samples obtained from day 3 and subsequently on the tip of umbilical venous catheter from day 6. Because of this, intravenous arbekacin was added to the vancomycin monotherapy. There were signs of progressive pneumonia on serial chest x-rays, and MRSA was also isolated from tracheal aspirates. The PDA had completely closed after one cycle of intravenous ibuprofen treatment, but the size of intracardiac mass had increased on the 12th day, despite of the dual antibiotic treatment. MRSA was cultured in five serial blood samples and in three serial tracheal aspirates.
The total durations of vancomycin and arbekacin treatment were 6 and 2 weeks, respectively. The initial dose of vancomycin was 10 mg/kg/dose, with a dose interval of 18 hours. This dosage was gradually increased to 30 mg/kg/18 hr by the 12th day because of the increased size of the cardiac vegetation, persistent MRSA septicemia, and clinical deterioration. The recommended serum trough level of vancomycin is 10–15 µg/mL5) for pediatric endocarditis. The serum trough level was 27 µg/mL on the 25th day of birth, and accordingly the dosage of vancomycin was decreased from 30 mg/kg/dose to 20 mg/kg/dose every 18 hours. Despite the dose adjustment, vancomycin trough level was still elevated to 21 µg/mL on the 32th day, and the dosage was reduced again to 10 mg/kg/dose every 18 hours. Following this, the serum trough levels were 16.7 µg/mL on the 38th day. The 10 mg/kg/18 hr vancomycin dosage was maintained until the end of the antibiotic treatment on day 52 of life. There was no evidence of nephrotoxicity in laboratory study results, and urine output had been adequate from birth to discharge. Automated auditory brainstem response was within normal range bilaterally at follow-up outpatient visit.
On serial echocardiograms, the right atrial mass began to shrink on 28th day of life (22 days after the initiation of antibiotic treatment), and there was no evidence of vegetation by 44th day (Fig. 2B). Subsequent follow-up studies did not identify any recurrence of vegetation on echocardiogram nor MRSA in serial blood cultures. A peripherally inserted central venous catheter (PCVC) had been placed on the day umbilical venous catheter was removed and was removed on the 31st day. A second PCVC was reintroduced on 43rd day of life for supplemental parenteral nutrition. This PCVC was removed on the 49th day of his age.
The patient was discharged on 72nd day of life with a mild degree of bronchopulmonary dysplasia, which did not require home oxygen therapy, and with a mild retinopathy of prematurity which did not need an intervention. He is meeting the normal growth and development milestones according to the age adjusted to his prematurity, and there is no evidence of recurrent intracardiac mass on out-patient echocardiograms.
The clinical signs of neonatal endocarditis are cardiac murmur or changing murmur, thrombocytopenia, microscopic hematuria, and skin lesions1,2,6). However, these signs are neither sensitive nor specific when considering other infectious diseases in neonates7). Echocardiogram is important not only in early diagnosis of congenital heart diseases but also detection of infective endocarditis, especially in the presence of septicemia and clinical deterioration.
In this report, we have presented a rare case of successfully treated MRSA endocarditis in an ELBW infant. To the best of our knowledge, there has only been a single report of an ELBW infant who had survived a MRSA endocarditis2). In that 1988 report, the endocarditis was diagnosed by echocardiogram on 25th day of life, and the patient was treated with vancomycin and rifampicin duotherapy. The patient had total resolution of the vegetation on serial echocardiograms and was reported to follow the normal course of development without major complications. In addition, several cases of successfully treated infective endocarditis have been reported in premature infants, with respective infectious agents of methicillin-sensitive S. aureus, group B Streptococcus, or candida species6,7,8). Prior to our experience, the most recent case of MRSA endocarditis in ELBW infant was reported in 20089), in which the patient had expired on 64th day of life from cardiopulmonary failure.
This case report is significant not only in view of successful treatment of MRSA endocarditis in an ELBW infant but also in demonstrating the importance of early detection of infective endocarditis as the focus of persistent septisemia in a premature infant. The atrial vegetation had been found on an echocardiogram, which had been performed to evaluate the PDA and as part of a survey to identify the primary focus of the ongoing septicemia.
According to the recent recommendations of the American Academy of Pediatrics' committee on infectious diseases, empirical antibiotic therapy of health care-associated MRSA infection includes vancomycin plus gentamicin10). Because the antibiotic susceptibility test of MRSA in our center has shown high rates of resistance to gentamicin, we chose other aminoglycoside, arbekacin as an alternative agent, based on the previous study about the successful treatment of MRSA infection of neonates and infants with arbekacin without significant side effects11). This is the first report of successful MRSA infective endocarditis of preterm infant treated with the antibiotic regimen of arbekacin with vancomycin.
Adequate choice of antibiotic agent and proper dosage are crucial for successful treatment and favorable prognosis of infective endocarditis. In neonates, it is especially important to check for possible side effects of high dose antibiotic therapy. In our case, the serum vancomycin trough level was checked weekly during the 6 weeks of vancomycin therapy, revealing toxic levels of serum vancomycin which required dose adjustment as well as monitoring for signs of end-organ damage. Despite this, the patient experienced no vancomycin-related complications as evidenced by normal urine output, serum creatinine levels, and automated auditory brainstem response.
Conflicts of interest
Conflict of interest:
No potential conflict of interest relevant to this article was reported.
References
1. Oelberg DG, Fisher DJ, Gross DM, Denson SE, Adcock EW 3rd. Endocarditis in high-risk neonates. Pediatrics 1983;71:392–397.



3. Verhoef J, Fleer A. Staphylococcus epidermidis endocarditis and Staphylococcus epidermidis infection in an intensive care unit. Scand J Infect Dis Suppl 1983;41:56–64.

4. Millard DD, Shulman ST. The changing spectrum of neonatal endocarditis. Clin Perinatol 1988;15:587–608.


5. Benner KW, Worthington MA, Kimberlin DW, Hill K, Buckley K, Tofil NM. Correlation of vancomycin dosing to serum concentrations in pediatric patients: a retrospective database review. J Pediatr Pharmacol Ther 2009;14:86–93.




6. Armstrong D, Battin MR, Knight D, Skinner J. Staphylococcus aureus endocarditis in preterm neonates. Am J Perinatol 2002;19:247–251.


7. Poon WB, Lian WB. Recurrent group B streptococcal septicemia in a very low birth weight infant with infective endocarditis and submandibular cellulitis. Ann Acad Med Singapore 2010;39:936–932.


8. Aydemir C, Erdeve O, Oguz SS, Altug N, Dilmen U. Successful treatment of Candida albicans endocarditis vegetations with recombinant tissue plasminogen activator in an extremely low birth weight preterm infant. Mycoses 2011;54:e590–e592.


9. Marks KA, Zucker N, Kapelushnik J, Karplus M, Levitas A. Infective endocarditis successfully treated in extremely low birth weight infants with recombinant tissue plasminogen activator. Pediatrics 2002;109:153–158.


10. American Academy of Pediatrics Committee on Infectious Diseases. Staphylococcal infections. Pickering LK, Baker CJ, Kimberlin DW, Long SS, editors. Red Book: 2012 Report of the Committee on Infectious Diseases. 29th ed. Elk Grove Village (IL): American Academy of Pediatrics, 2012;:653–667.
Fig. 1
The umbilical venous catheter was inserted on the 1st day after birth, with the catheter tip in the right atrium.
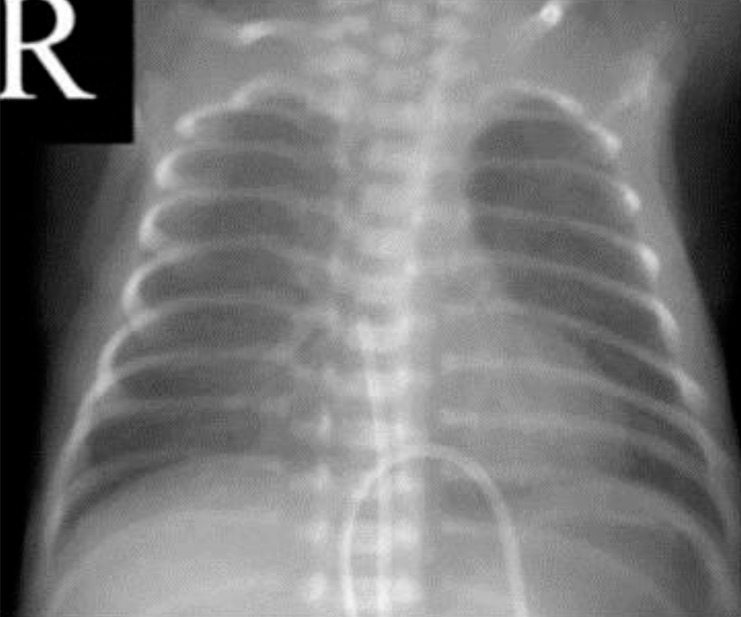
Fig. 2
(A) An echocardiogram on the 9th day after birth revealed a 0.25-cm2 oscillating mass (arrow) in the right atrium. The mass faced the atrial septum and the tricuspid valve. (B) The mass was completely resolved on the follow-up echocardiogram taken on the 44th day after birth. RA, right atrium; RV, right ventricle; LA, left atrium; LV, left ventricle.



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link PubMed
PubMed Download Citation
Download Citation


