Article Contents
| Korean J Pediatr > Volume 59(4); 2016 |
|
Abstract
Purpose
To identify the effects of modified parenteral nutrition (PN) and enteral nutrition (EN) regimens on the growth of very low birth weight (VLBW) infants.
Methods
The study included VLBW infants weighing <1,500 g, admitted to Chungnam National University Hospital between October 2010 and April 2014, who were alive at the time of discharge. Subjects were divided according to 3 periods: period 1 (n=37); prior to the PN and EN regimen being modified, period 2 (n=50); following the PN-only regimen modification, period 3 (n=37); following both PN and EN regimen modification. The modified PN regimen provided 3 g/kg/day of protein and 1 g/kg/day of lipid on the first day of life. The modified EN regimen provided 3.5-4.5 g/kg/day of protein and 150 kcal/kg/day of energy. We investigated growth rate, anthropometric measurements at 40 weeks postconceptional age (PCA) and the incidence of extrauterine growth restriction (EUGR) at 40 weeks PCA.
Results
Across the 3 periods, clinical characteristics, including gestational age, anthropometric measurements at birth, multiple births, sex, Apgar score, surfactant use and PDA treatment, were similar. Growth rates for weight and height, from time of full enteral feeding to 40 weeks PCA, were higher in period 3. Anthropometric measurements at 40 weeks PCA were greatest in period 3. Incidence of weight, height and head circumference EUGR at 40 weeks PCA decreased in period 3.
With the improvement of medical care for preterm infants during the past few decades, mortality and morbidity in preterm infants have gradually been decreasing1). Most studies have previously focused on reducing the major complications in preterm infants, especially very low birth weight (VLBW) infants. However, in recent years, many reports have focused on the nutrition and growth of preterm infants, which have previously been regarded as less important.
After birth, the nutrition supply of preterm infants is dependent on the external supply. As many preterm infants are not provided appropriate nutrients, they experience serious caloric and nutritional deficit. Therefore, they cannot accumulate sufficient nutrients suitable for their gestational age and cannot maintain their growth rate in uterus. As a result, they have difficulties in sustaining appropriate growth and achieving neurological and cognitive development2,3,4,5). Extrauterine growth restriction (EUGR) means that the preterm infants did not achieve the expected growth in the uterus after birth and is defined as a having weight, height and head circumference value less than the 10th percentile of the postconceptional age (PCA) and gender3). EUGR is a serious problem frequently occurring in preterm infants hospitalized in the neonatal intensive care unit (NICU). Most studies aiming to improve this problem emphasize the importance of nutrition6,7,8).
Recent studies aiming to improve the postnatal growth in preterm infants emphasize the proper supply of protein and lipid along with carbohydrate; this is primarily accomplished through parenteral nutrition (PN) as soon as possible in the early stages after birth, and it is reported that supplying more protein in the early stages is helpful for growth7,9,10). It is also reported that in case of enteral nutrition (EN), supplying higher amount of protein and calories is helpful for long-term growth11,12,13,14). However, there has been no study on whether the combined improvement of both PN and EN has any effect on growth. Therefore, the purpose of this study were; (1) to modify our PN and EN protocols sequentially based on the recent suggestions from other studies and literatures: (2) to evaluate the clinical impact of modified nutrition protocol on the postnatal growth and EUGR in VLBW infants.
Subjects included all VLBW infants less than 34 weeks of gestational age and a birth weight less than 1,500 g who were admitted in the NICU of Chungnam National University Hospital (CNUH) between October 2010 and April 2014 (3 years and 7 months). The study protocol was approved by the CNUH Institutional Review Board. Subjects were excluded if they died during hospitalization, were transferred to other hospitals or had serious congenital malformation such as in the heart or gastrointestinal tract that required surgical treatment.
A total of 124 subjects were included in the study and were separated into 3 groups according to changes in the nutrition protocol: period 1 (October 2010.September 2011) was before the protocol of PN and EN was modified, period 2 (October 2011-December 2012) was when the protocol of PN had been modified but the change of EN was trivial and period 3 (January 2013-April 2014) was after the protocol of both PN and EN were modified.
On the first day of life, total fluid volume was started at 60 mL/kg/day, and the amount was adjusted according to the insensible water loss, urine output and the required amount of fluid. The supply of carbohydrate was started with a glucose infusion rate of 4–6 mg/kg/min and was increased up to 10–15 mg/kg/min based on the blood glucose level. For the supply of protein, a 10% amino acid solution (PRIMEN INJ 10% 100 mL, Baxter Healthcare GmbH, Vienna, Austria) containing the essential amino acids was used. For the supply of lipid, a 20% lipid solution (SMOFLIPID 20% INJ 250 mL, Fresenius Kabi Austria GmbH, Graz, Austria) containing essential fatty acids and omega-3-fatty acids was used.
The modified PN regimen was as follows: (1) supply of protein was started at a daily dose of 3 g/kg, increased by 0.5-1 g/kg everyday up to 4 g/kg/day; (2) supply of lipid was started at 1 g/kg/day on the first day of life, increased by 0.5 g/kg/day to up to 3.5 g/kg/day. PN was continued until the target amount of enteral feeding was achieved and then adding of nutritional supplements was began; during period 1, the time of addition was when the amount of feeding reached 120 mL/kg/day, and during periods 2 and 3, it was when the amount of feeding reached 100 mL/kg/day.
Minimum enteral feeding was started at 10-15 mL/kg/day as soon as possible. The amount of feeding increased 10-30 mL/kg daily; the amount and significance of residual milk or feeding intolerance were checked using physical examination and abdomen radiograph. Human milk was the preferred feeding but when it was not available, commercial formula for preterm infants (Absolute baby well premi baby formula, Maeil Dairy Industry Co., Ltd., Seoul, Korea) was used. Macronutrient composition of human milk was calculated as follows: protein, 1 g/100 mL; lipid, 3.9 g/100 mL; carbohydrate, 7.2 g/100 mL15. Composition of the preterm formula was determined based on the nutrition facts reported by the manufacturers (http://absolute.maeili.com/absolute/01product/babywell03.jsp).
When the babies achieved their full enteral feeding to supply the targeted amount of protein and calories, commercial human milk fortifier (HMF) (Absolute baby well HMF, Maeil Dairy Industry Co., Ltd.) and protein supplement (PROMAX, Korea Medical Food Co., Ltd., Seoul, Korea) were added according to their feeding methods the macronutrient composition in each supplement was based on to the nutrition facts reported by each manufacturer (Absolute baby well HMF: http://absolute.maeili.com/absolute/01product/babywell06.jsp, PROMAX: http://www.medifoods.co.kr/sub/view_product.php?Code=0916-843&CatNo=22). One pack of HMF was used for every 60-120 mL of human milk and the concentration of the fortifier was adjusted so that it did not exceed 15%. The amount of protein supplement was also adjusted according to the targeted daily protein supply.
The final modified EN regimen was aimed to provide 3.5-4.5 g/kg/day of protein and 150 kcal/kg/day of total calorie.
To compare the clinical characteristics, we investigated the gestational age, anthropometric measurement at birth, incidence of multiple births, sex, Apgar score at 1 and 5 minutes after birth, prenatal use of steroid, surfactant use and patent ductus arteriosus (PDA) treatment. Measures of weight, height and head circumference at birth were retrieved from the medical records.
We also investigated the time of starting enteral feeding, feeding methods, time of adding supplements, PN duration, degree of maximum weight loss and age of regaining birth weight since those have been known as related factors to postnatal growth.
We estimated the average daily increments of weight, height and head circumference during PN before full enteral feeding and the average growth rate of weight, height and head circumference from the age of full enteral feeding to the 40 weeks PCA. We also identified weight, height and head circumference at 40 weeks PCA and the incidence of EUGR at 36 weeks PCA and 40 weeks PCA according to weight, height and head circumference. Using Fenton's growth curve, EUGR was defined as each growth value less than the 10th percentile for the PCA.
As the discharge outcome, we investigated the total duration of hospitalization and PCA at the time of discharge. We also investigated the incidence of necrotizing enterocolitis (NEC), bronchopulmonary dysplasia (BPD), retinopathy of prematurity (ROP), intravetricular hemorrhage (IVH), periventricular leukomalacia(PVL), cholestasis and sepsis.
NEC was defined as a modified Bell's staging criteria higher than stage II 16). ROP was considered to be significant as higher than stage 3 or when laser photocoagulation or operational treatment was required 17). IVH was considered to be significant when bleeding according to the Papile classification was higher than grade 3 18). PVL was diagnosed using brain magnetic resonance imaging. Sepsis was defined when it satisfied the criteria of systemic inflammatory response syndrome and the pathogen was detected from the blood culture 19). Cholestasis was defined as direct bilirubin more than 2 mg/dL when PN was used for more than 2 weeks 20
21).
For statistical analysis, we used IBM SPSS statistics ver. 22.0 (SPSS Inc., Chicago, IL, USA), including the chi-square test and Fisher exact test for categorical variants, the Kruskal-Wallis test for serial variants, and Pearson coefficient for co-relationships. A Pvalue of 0.05 <was considered statistically significant.
Among the total 124 subjects, 37 were from period 1; 50 were from period 2; and 37 were from period 3. There were no significant differences among each period in terms of average gestational age, birth weight, height, head circumference, multiple births, sex, Apgar scores at 1 and 5 minutes after birth, use of surfactant and treatment of PDA. The use of antenatal steroid was higher in periods 2 and 3 compared to that in period 1 (P=0.035).
The time of starting enteral feeding showed a tendency to be faster in periods 2 and 3 compared to that in period 1 (P=0.051). The rate of human milk feeding was no significant differences among each period. The time of adding extra protein and or HMF became significantly faster in periods 2 and 3 than in that in period 1, as was intended (31.0±16.4 vs. 25.5±14.6 vs. 23.1±13.6 day of life, respectively, P=0.047). The duration of PN had a nonsignificant tendency to be shorter in periods 2 and 3 than that in period 1.
The degree of maximum weight loss was significantly lower in periods 2 and 3 than in period 1 (13.4±5.1% vs. 12.5±5.3% vs. 7.2±3.0%, respectively,P=0.000) and the time to regain birth weight was significantly shorter in periods 2 and 3 than in period 1 (17.7±8.3 days vs. 14.3±5.3 days vs. 11.1±3.5 days, respectively,P=0.000)
There were no significant differences among each period in the growth rates of average weight, height and head circumference during PN before full enteral feedingFig. 1. The average growth rate of weight from the time of full enteral feeding to 40 weeks PCA was significantly higher in period 3 compared to that in period 1 and period 2 (P=0.003). The average growth rate of height from the time of full enteral feeding to 40 weeks PCA also significantly increased (P=0.011). The growth rate of head circumference from full enteral feeding to 40 weeks PCA showed no significant difference (P=0.134) (Fig. 2)
There were significant increases in weight at 40 weeks PCA in periods 2 and 3 compared to that in period 1 (P=0.000). Height (P=0.000) and head circumference (P=0.000) at 40 weeks PCA also were significantly greater in periods 2 and 3 compared to those in period 1 (Fig. 3).
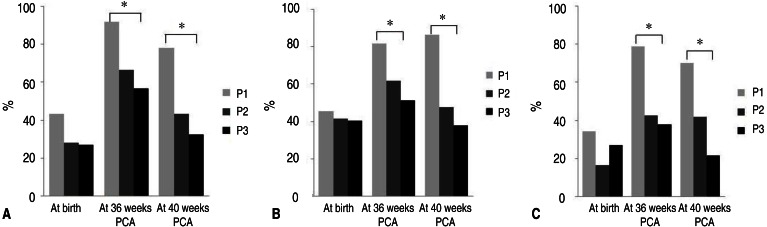
The incidence of birth weight below the 10th percentile for gestational age showed no significant differences among periods (43.2% vs. 28% vs. 27%, respectively, P=0.232). The incidence of weight EUGR at 36 weeks PCA was significantly lower in period 2 (66.6%) and period 3 (56.8%) than in period 1 (91.9%; P= 0.002). The incidence of weight EUGR at 40 weeks PCA was also significantly lower in period 2 (43.2%) and period 3 (32.4%) than in period 1 (78.1%; P=0.000) (Fig. 4A).
The incidence of birth height below the 10th percentile for gestational age showed no significant differences among different periods (45.5% vs. 41.7% vs. 40.5%, respectively, P=0.910). The incidence of height EUGR at 36 weeks PCA was significantly lower in period 2 (61.7%) and period 3 (51.4%) than in period 1 (81.8%; P=0.002). The incidence of height EUGR at 40 weeks PCA was also significantly lower in period 2 (47.6%) and period 3 (37.8%) than in period 1 (86.2%; P=0.000) (Fig. 4B).
The incidence of having head circumference at birth below the 10th percentile for gestational age showed no significant differences among different periods (34.4% vs. 16.7% vs. 27%, respectively, P=0.185). The incidence of head circumference EUGR at 36 weeks PCA was significantly lower in period 2 (42.6 %) and period 3 (37.8%) than in period 1 (78.8%; P=0.001). The incidence of head circumference EUGR at 40 weeks PCA was significantly lower in period 2 (41.9%) and period 3 (21.6%) than in period 1 (70.0%; P=0.000) (Fig. 4C).
The duration of hospitalization decreased in periods 2 and 3 compared to that in period 1 (76.8±29.5 days vs. 68.9±21.4 days vs. 61.1±20.3 days, respectively, P=0.029), and PCA at time of discharge was significantly lesser in periods 2 and 3 than in period 1 (40+4±2+6 weeks vs. 38+5±2+2 weeks vs. 38+1±1+4 weeks, respectively, P=0.000).The prevalence of diseases such as NEC, BPD, ROP, IVH, PVL, cholestasis and sepsis were no significant differences.
The purpose of this study was to determine the effects of nutrition on the postnatal growth in the VLBW infants by modifying PN and EN protocol. We found that after application of modified protocol, anthropometric measurement including weight, height and head circumference at 40 weeks PCA increased and the incidence of EUGR at 36 weeks and 40 weeks PCA decreased.
As the survival rate of VLBW infants increases, the occurrence of morbidities has received increasing attention over survival. In recent years, long-term growth and neurodevelopment outcomes have been regarded as important issues. Accordingly, the clinical studies about EUGR, which is closely related with growth and neurodevelopment outcomes, are important. Several studies have indicated that inappropriate nutrient supply during hospitalization in the NICU is one of the major causes of EUGR and that to reduce this problem, proper nutrition supply must be emphasized6,7,8).
Firstly, we modified the PN protocol, which is a major source of nutrition in the early stage after birth, and applied it to the VLBW infants (period 2).
Denne and Poindexter22) reported that insufficient supply of protein in the early stage after birth is related to growth failure and that earlier intravenous supply of higher amounts of amino acids could prevent the shortage of protein in the early stage after birth and help improve the growth rate and long-term neurodevelopment outcome. Thureen et al.9)reported that they could reduce the incidence of EUGR without any adverse effects when they supplied 3 g/kg/day of protein from the first day of PN compared with when they supplied 1 g/kg/day. On the basis of these studies, recent nutrition guidelines recommend that protein supply of 3 g/kg/day be started from the first day after birth7) , and in our study we modified our PN protocol to supply 3 g/kg/day of protein from the first day. In addition, Van Aerde et al.23) reported that delayed supply of lipids causes deficiency of essential fatty acids and results in a decrease of body weight and brain weight. Dit Trolli et al.10) reported that as the total cumulated amount of lipids for premature infants less than 28 weeks in the first 14 days increased, better neurological outcomes were obtained when they reached 1 year of age. Based on these studies, it is currently recommended that lipid supply be started from 0.5-1 g/kg/day on the first day of birth and increased up to 3.0-3.5 g/kg/day7). In our study, we started to supply lipids on the first day rather than the second day, increasing the maximum supply up to 3.5 g/kg/day.
Although PN is used as the major source of nutrients in the early unstable stage after birth, but eventually EN is the major source of nutrients for further growth. Because the period of EN was longer than that of PN by approximately 1.5-2 folds during hospitalization, an additional study was carried out on the improvement of EN (period 3).
Because it has been reported that human milk is often unable to supply all the necessary nutrients for the preterm infants despite of its various advantages, HMF should be applied24). Additionally, Arslanoglu et al.25) reported that even when fortified human milk is supplemented, because the ingredients of the milk change over time, it is not possible to measure the exact amount of calories or nutrients supplied to premature infants, and a less lower amount of protein and calories can be supplied than originally intended. These aspects can cause growth failure. In a systemic review executed by the Cochrane collaboration, it was reported that premature infants nourished with 3-4 g/kg/day of protein showed improved growth compared to those nourished with less than 3 g/kg/day of protein26), and according to the recommendation released in 2010 by the European Society for Pediatric Gastroenterology, Hepatology and Nutrition, premature infants with birth weight less than 1,200 g need 4.0-4.5 g/kg/day of protein and those with birth weight more than 1,200 g need 3.5-4 g/kg/day and these supplies of high protein have advantageous effects on catch-up growth27). Based on these studies, we modified our EN protocol based on increasing the targeted protein supply by 3.5-4.5 g/kg/day and increasing the targeted caloric intake by 150 kcal/kg/day. Because of the commercial HMF could not supply the amount of target protein even if it is used with a maximum concentration, we added an extra protein supplement to supply the amount of target protein.
Although achieving catch-up growth at an early stage after birth is a very important factor in long-term growth and neurodevelopment outcomes, there are concerns that excessively rapid catch-up growth can lead to long-term metabolic disorders such as obesity, impaired glucose intolerance, hypertension and cardiovascular disorders28,29). However, we did not allow over growth with monitoring growth rate.
When we compared the clinical characteristics of subjects in this study, prenatal use of steroids gradually but significantly increased from period 1 through period 3, possibly because of the change of management policy for preterm birth. Additionally, the starting point of feeding became earlier in periods 2 and 3. In the past, because of early feeding was thought to increase the risk of NEC, delayed feeding was recommended. However, recent studies reported that early start of feeding has various advantages such as reducing the risk of NEC, shortening the total duration of PN and reducing the risk of sepsis, and it is recommended that minimal EN be started as early as possible30,31). Therefore, the change in the starting time of feeding is thought to reflect these changes in management trends. In our study, the time of both starting enteral feeding and adding of HMF and other nutritional supplements became faster in periods 2 and 3 than in period 1 as originally intended in the modification of the nutrition guidelines, and the finding indicates that early start of feeding resulted in earlier full enteral feeding.
Firstly, in order to evaluate the effect of PN modification only, we compared the factors related to growth of periods 1 and 2 after applying the modified PN protocol. As a result, the time needed to recover the birth weight was shorten, weight, height and head circumference all increased at 40 weeks PCA and the incidence of weight, height, and head circumference EUGR decreased. Because we changed the protein and lipids altogether, our approach was somewhat different from that of studies that adjusted protein only, but the results of our study showed that the earlier start of PN with higher protein and calorie improves growth similarly to the aforementioned studies9,22).
In several studies that compared the growth of premature infants after supplying different amounts of protein, growth rate was improved in preterm infants that received more protein12,13). Our study showed a similar result when comparing periods 2 and 3 after modifying both PN and EN; the growth rates for weight and height from the full enteral feeding day to 40 weeks PCA increased. Additionally, the percentage of maximum weight loss decreased, and the time needed to recover the birth weight also shortened. Although the difference was not significant, the incidence of weight, height and head circumference EUGR tended to decrease. These results show that the modified EN is helpful to improve postnatal growth failure not resolved by modifying PN only. When analyzing the results comprehensively, all anthropometric measurement at 40 weeks PCA were greatest in period 3 and the incidence of weight, height and head circumference EUGR at 36 and at 40 weeks were the lowest in period 3. The growth improvement in 40 weeks PCA was pronounced than in 36 weeks PCA. In period 3, despite growth rate of head circumference was not improved, head circumference at 40 weeks PCA was improved and the incidence of head circumference EUGR at 40 weeks PCA was significantly decreased. These results were thought to be associated with increased growth rate during EN, decreased maximum weight loss and faster recovery of birth weight. Moreover, in order to improve the postnatal growth in VLBW infants, PN modification is important but also EN modification is more important.
Lemons et al.32) reported that the incidence of weight EUGR at 36 weeks PCA was as high as 99% among infants with a birth weight less than 1,000 g and 97% among those with a birth weight less than 1,500 g, and Kim et al.33) reported that the incidence of weight EUGR at the time of discharge was still 67% among infants with a birth weight less than 1,500 g, which implies that EUGR is a frequent phenomenon in the NICU. In our study, in period 1, the incidence of weight below 10th percentile at birth was 43.2%, but it increased to 91.9% at 36 weeks PCA and 78.1% at 40 weeks PCA. And in ELBW infants, the incidences of weight EUGR at 36 and 40 weeks PCA were 100% and 87.5%, respectively, in period 1 (data not shown), indicating high EUGR prevalence.
After PN and EN modification, the incidence of weight EUGR at 40 weeks PCA was reduced to 43.2% in period 2 and 32.4% in period 3. Especially in period 3, the incidence of weight EUGR at 40 weeks PCA was similar to the incidence of less than 10th percentile of weight at birth (27%). This similarity suggests the possibility of that the infants born with small weight for estational age might not achieve catch-up growth, however, whether the infants with weight EUGR at 40 weeks PCA were actually born with small weight for gestational age was not confirmed.
During study period with modifying both PN and EN, the duration of hospitalization decreased and PCA at time of discharge was significantly lesser. Regarding the prevalence of morbidities, the incidence of PVL tended to be higher in period 3 but there was no significance. One study reported that early amino acid supply could decrease the incidence of PVL, which in turn may be associated with good neurodevelopment outcomes34). Since the development of PVL is known to be associated with multiple factors such as IVH, hypocarbia, maternal preterm premature rupture of membranes and maternal preeclampsia35), it is possible that the higher incidence of PVL in period 3 in the present study might be resulted from factors other than nutrition. The prevalence of other diseases such as NEC, BPD, ROP, PVL, cholestasis and sepsis were no significant differences.
To evaluate the protein and caloric stability of the PN, we took blood samples twice weekly to evaluate the levels of blood urea nitrogen (BUN), base deficit and triglyceride (TG). The stability of EN was checked using periodic blood tests at 1- to 2-week intervals. The peak BUN and base deficit tended to higher in period 2. However, they were within the limits allowed in preterm infants. Despite supply more lipids in periods 2 and 3, the peak TG level of periods 2 and 3 was not high compare to that of period 1 (data not shown).
There are several limitations in our study. Historical case control study is a major drawback. And it was carried out at a single center with a small number of subjects and we did not perform follow-up observations of long-term outcomes of growth and neurodevelopment.
Despite these limitations, the important strength of our study is that the improvement of both PN and EN improve the postnatal growth and reduce the incidence of EUGR. The role of EN is especially important for postnatal growth in VLBW infants and we suggest that supply of higher protein and calorie during EN are advantageous.
Despite of the improvement of both PN and EN, in some VLBW infants, there was no distinct improvement of growth. It indicates that for some preterm infants, the change of nutrition alone is not sufficient for catch-up growth. Further studies are required to find the additional causes and solutions of EUGR.
Acknowledgments
This study was presented at 64th Annual Autumn Meeting of The Korean Pediatrics Society (2014).
Conflicts of interest
Conflict of interest:
No potential conflict of interest relevant to this article was reported.
References
1. Hahn WH, Chang JY, Chang YS, Shim KS, Bae CW. Recent trends in neonatal mortality in very low birth weight Korean infants: in comparison with Japan and the USA. J Korean Med Sci 2011;26:467–473.



2. Bloom BT, Mulligan J, Arnold C, Ellis S, Moffitt S, Rivera A, et al. Improving growth of very low birth weight infants in the first 28 days. Pediatrics 2003;112(1 Pt 1): 8–14.


3. Clark RH, Thomas P, Peabody J. Extrauterine growth restriction remains a serious problem in prematurely born neonates. Pediatrics 2003;111(5 Pt 1): 986–990.


4. Berry MA, Abrahamowicz M, Usher RH. Factors associated with growth of extremely premature infants during initial hospitalization. Pediatrics 1997;100:640–646.


5. Embleton NE, Pang N, Cooke RJ. Postnatal malnutrition and growth retardation: an inevitable consequence of current recommendations in preterm infants. Pediatrics 2001;107:270–273.


6. Dusick AM, Poindexter BB, Ehrenkranz RA, Lemons JA. Growth failure in the preterm infant: can we catch up? Semin Perinatol 2003;27:302–310.


7. Ehrenkranz RA. Early, aggressive nutritional management for very low birth weight infants: what is the evidence? Semin Perinatol 2007;31:48–55.


8. Ehrenkranz RA. Early nutritional support and outcomes in ELBW infants. Early Hum Dev 2010;86(Suppl 1): S21–S25.
9. Thureen PJ, Melara D, Fennessey PV, Hay WW Jr. Effect of low versus high intravenous amino acid intake on very low birth weight infants in the early neonatal period. Pediatr Res 2003;53:24–32.


10. dit Trolli SE, Kermorvant-Duchemin E, Huon C, Bremond-Gignac D, Lapillonne A. Early lipid supply and neurological development at one year in very low birth weight (VLBW) preterm infants. Early Hum Dev 2012;88(Suppl 1): S25–S29.

11. Collins CT, Chua MC, Rajadurai VS, McPhee AJ, Miller LN, Gibson RA, et al. Higher protein and energy intake is associated with increased weight gain in pre-term infants. J Paediatr Child Health 2010;46:96–102.


12. Cooke R, Embleton N, Rigo J, Carrie A, Haschke F, Ziegler E. High protein pre-term infant formula: effect on nutrient balance, metabolic status and growth. Pediatr Res 2006;59:265–270.


13. Costa-Orvay JA, Figueras-Aloy J, Romera G, Closa-Monasterolo R, Carbonell-Estrany X. The effects of varying protein and energy intakes on the growth and body composition of very low birth weight infants. Nutr J 2011;10:140



14. Hsiao CC, Tsai ML, Chen CC, Lin HC. Early optimal nutrition improves neurodevelopmental outcomes for very preterm infants. Nutr Rev 2014;72:532–540.


15. Hong CE. Textbook of pediatrics. 9th ed. Seoul: Korea Textbook Publishing Co., 2008.
16. Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall R, Barton L, et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann Surg 1978;187:1–7.



17. International Committee for the Classification of Retinopathy of Prematurity. The International Classification of Retinopathy of Prematurity revisited. Arch Ophthalmol 2005;123:991–999.


18. Dammann O, Leviton A. Duration of transient hyperechoic images of white matter in very-low-birthweight infants: a proposed classification. Dev Med Child Neurol 1997;39:2–5.


19. Martin RJ, Fanaroff AA, Walsh MC. Neonatal-perinatal medicine: diseases of the fetus and infant. 9th ed. St. Louis (MO): Saunders/Elsevier, 2010.
20. Kubota A, Yonekura T, Hoki M, Oyanagi H, Kawahara H, Yagi M, et al. Total parenteral nutrition-associated intrahepatic cholestasis in infants: 25 years' experience. J Pediatr Surg 2000;35:1049–1051.


21. Park KP, Kim SY, Kim HM. Total parenteral nutrition-associated cholestasis in premature infants. J Korean Pediatr Soc 2003;46:17–23.
22. Denne SC, Poindexter BB. Evidence supporting early nutritional support with parenteral amino acid infusion. Semin Perinatol 2007;31:56–60.


23. Van Aerde JE, Wilke MS, Feldman M, Clandinin MT. editors. Accretion of lipid in the fetus and newborn. Polin RA, Fox WW, editors. Fetal and neonatal physiology. 2nd ed. Philadelphia: Saunders, 1998;:454–470.
25. Arslanoglu S, Moro GE, Ziegler EE. Preterm infants fed fortified human milk receive less protein than they need. J Perinatol 2009;29:489–492.


26. Premji SS, Fenton TR, Sauve RS. Higher versus lower protein intake in formula-fed low birth weight infants. Cochrane Database Syst Rev 2006;(1): CD003959

27. Agostoni C, Buonocore G, Carnielli VP, De Curtis M, Darmaun D, Decsi T, et al. Enteral nutrient supply for preterm infants: commentary from the European Society of Paediatric Gastroenterology, Hepatology and Nutrition Committee on Nutrition. J Pediatr Gastroenterol Nutr 2010;50:85–91.


28. Thureen PJ. The neonatologist's dilemma: catch-up growth or beneficial undernutrition in very low birth weight infants-what are optimal growth rates? J Pediatr Gastroenterol Nutr 2007;45(Suppl 3): S147–S151.


29. Greer FR. Long-term adverse outcomes of low birth weight, increased somatic growth rates, and alterations of body composition in the premature infant: review of the evidence. J Pediatr Gastroenterol Nutr 2007;45(Suppl 3): S147–S151.


30. Hartel C, Haase B, Browning-Carmo K, Gebauer C, Kattner E, Kribs A, et al. Does the enteral feeding advancement affect short-term outcomes in very low birth weight infants? J Pediatr Gastroenterol Nutr 2009;48:464–470.


31. Chauhan M, Henderson G, McGuire W. Enteral feeding for very low birth weight infants: reducing the risk of necrotising enterocolitis. Arch Dis Child Fetal Neonatal Ed 2008;93:F162–F166.


32. Lemons JA, Bauer CR, Oh W, Korones SB, Papile LA, Stoll BJ, et al. Very low birth weight outcomes of the National Institute of Child health and human development neonatal research network, January 1995 through December 1996. NICHD Neonatal Research Network. Pediatrics 2001;107:E1


33. Kim ES, Sohn JA, Lee EH, Choi EJ, Lee HJ, Lee JA, et al. Extrauterine growth restriction in very low birth weight infants. J Korean Soc Neonatol 2010;17:53–63.
Fig. 1
The average growth rates of weight (A), height (B), and head circumference (C) during parenteral nutrition before full enteral feeding. There were no significant differences between periods.
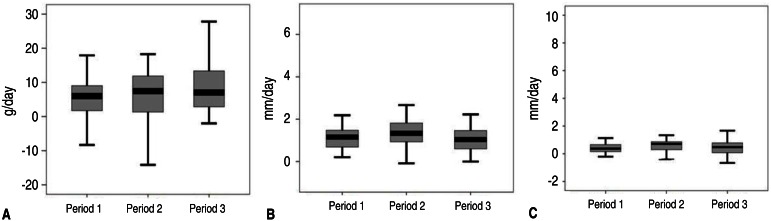
Fig. 2
The average growth rates of weight (A), height (B), and head circumference (C) from full enteral feeding to 40 weeks postconceptional age. *P<0.05, significant difference between periods.
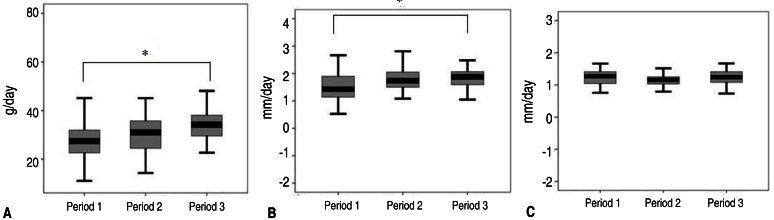
Fig. 3
Anthropometric measurements of weight (A), height (B), and head circumference (C) at 40 weeks postconceptional age. *P<0.05, significant difference between groups.

Fig. 4
The incidence of EUGR at 36 and 40 weeks PCA. (A) Incidence of weight EUGR is significantly decreased in periods 2 and 3. (B) Incidence of height EUGR is significantly decreased in periods 2 and 3. (C) Incidence of head circumference EUGR is significantly decreased in periods 2 and 3. EUGR, extrauterine growth restriction; PCA, postconceptional age. *P<0.05, significant difference between groups.



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link PubMed
PubMed Download Citation
Download Citation


