Article Contents
| Korean J Pediatr > Volume 60(4); 2017 |
|
Abstract
Purpose
The aims of this study were to compare serum procalcitonin (PCT) levels between febrile children with Kawasaki disease (KD) and those with bacterial or viral infections, and assess the clinical usefulness of PCT level in predicting KD.
Methods
Serum PCT levels were examined in febrile pediatric patients admitted between August 2013 and August 2014. The patients were divided into 3 groups as follows: 49 with KD, 111 with viral infections, and 24 with bacterial infections.
Results
The mean PCT level in the KD group was significantly lower than that in the bacterial infection group (0.82±1.73 ng/mL vs. 3.11±6.10 ng/mL, P=0.002) and insignificantly different from that in the viral infection group (0.23±0.34 ng/mL,P=0.457). The mean erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) level in the KD group were significantly higher than those in the viral and bacterial infection groups (P<0.001 and P<0.001 for ESR, P<0.001 and P=0.005 for CRP, respectively). The proportion of patients in the KD group with PCT levels of >1.0 ng/mL was significantly higher in the nonresponders to the initial intravenous immunoglobulin treatment than in the responders (36% vs. 8%, P=0.01).
Procalcitonin (PCT), the prehormone of calcitonin, is released in response to proinflammatory stimuli, particularly bacteria-associated mediators1). Recently, PCT has been more frequently used as a biomarker of sepsis than C-reactive protein (CRP), another acute-phase reactant2). In a study of PCT levels in adult patients in various inflammatory states, PCT levels were found to be significantly elevated in patients with bacterial or fungal infections, but normal or slightly elevated in those with several inflammatory diseases3). Furthermore, in a study of PCT levels in pediatric patients, PCT was found to be a significant biomarker for diagnosing pediatric urinary tract infection and neonatal sepsis, and for identifying patients without serious bacterial infections in order to prevent antimicrobial overuse in the pediatric intensive care unit4,5,6).
Many children present to hospitals with fevers of varying etiologies. One of the most important causes of fever in children younger than 5 years is Kawasaki disease (KD). KD is a well-known systemic vasculitic disorder, and the systemic inflammation associated with the condition causes elevations in white blood cell (WBC) counts and CRP levels, in addition to elevation in the erythrocyte sedimentation rate (ESR)7,8). Before a diagnosis of KD is made and intravenous immunoglobulin (IVIG) therapy is initiated, many patients with KD receive antibiotic (oral/intravenous) therapy and a diagnosis of KD is delayed because high ESR and CRP levels are commonly thought to indicate bacterial infections.
The first study of PCT levels in patients with KD compared with PCT levels in patients with other diseases was reported in 20049). Although the clinical impact of serum PCT levels in patients with KD has not been determined, the relation between PCT and KD severity, and the comparison of PCT levels between complete and incomplete KD were also reported10,11).
The aim of this study was to report our experience about PCT levels in febrile children with KD and those with bacterial or viral infections, and the clinical usefulness of PCT level in predicting KD.
We evaluated the serum PCT levels of 400 febrile pediatric patients admitted from August 2013 to August 2014 at single tertiary center. Fever was defined as a body temperature of ≥38℃, and the age range of the enrolled patients was from 29 days to <5 years. The enrolled patients were divided into 3 groups—the KD, viral infection, and bacterial infection groups—according to the definitions described below.
Complete KD was diagnosed in cases in which a fever persisted for ≥5 days, and at least 4 of the 5 typical clinical manifestations were observed, based on the 2004 American Heart Association criteria7,8). We also included patients with incomplete KD, which was diagnosed when fewer than four of the 5 typical clinical manifestations were observed based on the criteria12). Responders to IVIG treatment included patients with KD whose fever subsided within 48 hours after the initial treatment, whereas nonresponders included patients with KD whose fever persisted beyond 48 hours and required additional IVIG treatment.
To identify viral pathogens, virus reverse transcription polymerase chain reaction (RT-PCR; 16 ACE Detection, Seegene, Seoul, Korea) was performed on nasal or throat swab specimens obtained on the day of admission. RT-PCR included tests for metapneumovirus, adenovirus (A–F), coronavirus 229E/OC43, parainfluenza virus 1/2/3, influenza A/B virus, rhinovirus, respiratory syncytial virus A/B, and bocavirus. Viral infections were diagnosed on the basis of positive RT-PCR results. Patients with clinical diagnostic features of viral infections, such as hand, foot, and mouth disease, or herpangina, were also included. Bacterial infections were confirmed by positive culture results in various samples from each patient (i.e., blood, urine, or cerebrospinal fluid). KD with viral infection confirmed by RT-PCR or bacterial infection confirmed by culture was excluded. We retrospectively reviewed the patients' characteristics and clinical courses by using medical records.
Peripheral blood samples were analyzed on the day of admission for measurement of markers including PCT. Serum PCT levels were measured by using electrochemiluminescence immunoassays (Elecsys BRAHMS PCT, Roche, Henningsdorf, Germany) with a detection range of 0.02–100 ng/mL. The following cutoff levels of PCT were used to determine whether antibiotic treatment would be used: PCT<0.25 ng/mL, antibiotics strongly discouraged; PCT<0.5 ng/mL, antibiotics discouraged; and PCT>1.0 ng/mL, antibiotics strongly encouraged13). We also obtained complete blood cell counts, ESR, and CRP levels. Serum CRP levels were measured by using latex-enhanced immunoturbidimetry (ADVIA Chemistry XPT System, Siemens, Erlangen, Germany).
This study was approved by the Institutional Review Board of the Keimyung University Dongsan Medical Center (approval number: 2015-01-003).
All statistical analyses were performed with IBM SPSS Statistics ver. 21.0 (IBM Co., Armonk, NY, USA), and collected data were described as frequencies and means with the standard deviations. The chi-square test was used for comparisons of categorical data, and 1-way analysis of variance followed by the Bonferroni post hoc test for multiple comparisons was used for comparisons of continuous data among the 3 groups. In patients with KD, the unpaired t test and logistic regression analysis were applied to compare the PCT, ESR, and CRP levels. A P value of <0.05 was considered statistically significant.
A total of 184 patients were enrolled in this study, including 49 with KD, 111 with viral infections, and 24 with bacterial infections. The characteristics of patients in each group are shown in Table 1.
KD and incomplete KD were observed in 40 and 9 patients, respectively. All patients with KD were initially treated with 2 g/kg IVIG and 60–100 mg/kg oral aspirin, and 38 patients were classified as responders and 11 patients as nonresponders.
Of the 111 patients with viral infections, 108 showed positive RT-PCR results. The most commonly detected virus was respiratory syncytial virus A/B (40 of 108, 37%), followed by rhinovirus (22 of 108, 20%) and parainfluenza (17 of 108, 16%). Thirteen patients (13 of 108, 12%) had mixed infection with 2 kinds of viruses. Among the patients with viral infection, the most common diagnosis was acute lower respiratory tract infection. Acute bronchiolitis was diagnosed in 65 patients (60%) and pneumonia in 18 patients (18 of 108, 17%), followed by croup in 8, influenza in 5, and asthma in 5 patients. The other diagnoses were bronchitis in 3; hand, foot, and mouth disease in 1; herpangina in 1; influenza in 1; and fever without localizing signs in 1.
Bacterial infections were confirmed in 10, 13, and 1 patient, respectively, on the basis of positive results of blood, urine, and both blood and urine cultures. Of the 24 patients with bacterial infections, the causative organisms were Staphylococcus or Streptococcus in blood, and Enterococcus, Escherichia coli, or Klebsiella pneumoniae in urine. The final diagnoses of bacterial infections were septicemia, pneumonia, cellulitis, and urinary tract infections.
The mean age of the KD group was significantly higher than that of the viral infection group (P=0.006). Patients in the KD group had a significantly higher mean body weight than those in the viral and bacterial infection groups (P=0.035 and P=0.014); however, the mean body mass index was not significantly different among the groups. The sex distributions were not different among the 3 groups. The duration of fever before admission was a mean of 4.4, 2.6, and 1.7 days in the KD, viral infection, and bacterial infection groups, respectively. Patients in the KD group had a significantly longer fever duration before admission than those in the viral and bacterial infection groups (P=0.001 and P<0.001). The bacterial infection group had a significantly longer duration of admission than the viral infection group (P=0.006).
The laboratory findings of all patients included in the study are shown in Table 2.
The mean WBC count in the KD and bacterial infection groups was significantly higher than that in the viral infection group (P=0.044 and P=0.005), and the mean neutrophil proportion in the KD group was significantly higher than that in the viral infection group (P<0.001). The mean ESR in the KD group was significantly higher than that in the viral and bacterial infection groups (P<0.001 and P<0.001). The mean CRP level in the KD infection group was significantly higher than that in the viral and bacterial groups (P<0.001 and P<0.001).
The mean PCT levels in the KD and viral and bacterial infection groups were 0.82±1.73, 0.23±0.34, and 3.11±6.10 ng/mL, respectively. The mean PCT level in the bacterial infection group was significantly higher than that in the KD and viral infection groups (P=0.002 and P<0.001). Although the mean PCT level in the KD group was higher than that in the viral infection group, this difference was not statistically significant (P=0.457).
The numbers of patients according to PCT levels in each group are shown in Table 2. The number of patients with PCT levels <0.25 ng/mL and >1.0 ng/mL was significantly different between the KD and viral infection groups (P=0.001) and between the viral infection and bacterial infection groups (P<0.001), but not between the KD and bacterial infection groups (P=0.099).
In the KD group, PCT levels were not significantly correlated with fever durations, ESR, or CRP levels (P=0.792, P=0.994, and P=0.516, respectively).
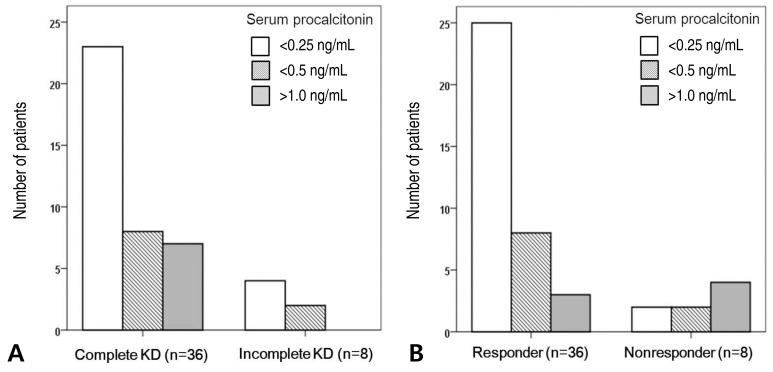
There was no significant difference in PCT levels between patients with complete and incomplete KD (P=0.477) (Fig. 1A). Among the nine patients with incomplete KD, only one had a PCT level >0.5 ng/mL.
Between IVIG responders and nonresponders among patients with KD, there was also no significant difference in PCT level, ESR, and CRP level (P=0.434, P=0.052, and P=0.107, respectively). However, after adjustment for age by using analysis of covariance (ANCOVA), a significant difference was observed in PCT and CRP between responders and nonresponders in the KD group (P=0.023 and P=0.012).
Of the 11 IVIG nonresponders, 2 (18%) had a PCT level <0.25 ng/mL and 4 (36%) had a PCT level >1.0 ng/mL. In comparison, of the 38 IVIG responders, 25 (66%) had a PCT level <0.25 ng/mL and 3 (8%) had a PCT level >1.0 ng/mL. A lower proportion of nonresponders than responders had PCT levels <0.25 ng/mL (P=0.01). A higher proportion of nonresponders than responders had PCT levels >1.0 ng/mL (P=0.01) (Fig. 1B).
The results of this study demonstrated that mean PCT level was beneficial in differentiating febrile children with KD from those with bacterial infections. Moreover, patients with KD with PCT levels >1.0 ng/mL tended to be nonresponders to initial IVIG treatment. In addition, ESR and CRP levels were useful in differentiating between patients with KD and those with viral infections.
Among many clinical markers of inflammation and sepsis, PCT is more accurate than CRP in differentiating bacterial infections from noninfectious inflammation1). This is because the peak PCT level is reached more rapidly than the peak CRP level, and the synthesis mechanisms of PCT and CRP are somewhat different. PCT is mainly produced in the liver and monocytes, and its secretion is modulated by lipopolysaccharides and sepsis-related cytokines such as tumor necrosis factor (TNF)-α. CRP is produced in the liver, mainly in response to interleukin (IL)-62,14). In pediatric research, PCT has been used in the study of neutropenia, fever in young infants, pediatric urinary tract infections, pediatric community-acquired pneumonia, and appendicitis4,15,16,17).
KD is a systemic vasculitic disorder that leads to endothelial cell activation and damage caused by increased levels of inflammatory cytokines such as TNF-α, IL-1, and IL-618). These inflammatory cytokines increase the levels of acute-phase reactants such as CRP and PCT. Increased CRP level in patients with KD plays a crucial role in diagnosis and treatment, and is also a risk factor for coronary artery-related complications12,19,20). However, the clinical impact of serum PCT levels in patients with KD has not been determined.
Fever is the first symptom to appear in KD, and the other symptoms do not usually develop simultaneously7,8). The initial high ESR and CRP levels associated with fever are suspected to be indicative of bacterial infection; hence, oral or intravenous antibiotic therapy may be administered, which usually continues until the symptoms fulfill the criteria of KD. However, patients with KD do not respond to antibiotic therapy and require IVIG treatment instead. Because KD is a difficult inflammatory disease to diagnose and PCT is a known biomarker of sepsis to diagnose bacterial infection and decide antibiotic treatment, we hypothesized that serum PCT level may differentiate KD from other infections.
The first study of PCT levels in patients with KD was reported in 2004. In this report, patients with KD had a higher mean PCT level than patients with viral and autoimmune diseases and healthy children, whereas patients with KD and those with bacterial infections showed a similar PCT level9). The present study also showed similar results: (1) The mean PCT level in patients with KD and in those with bacterial infection was significantly higher than that in patients with viral infection. (2) The mean PCT level in patients with KD was lower than that in patients with bacterial infection; however, this difference was not significant. We speculated that immune responses to bacterial infections may occur in patients with KD, although the causative microorganism of KD has not yet been confirmed. The definite cause of KD is currently unknown; however, it is generally accepted that KD develops as a result of a genetic predisposition combined with an infection with an undefined trigger or an autoimmune mechanism. Many bacterial and viral agents have been suggested but not confirmed as triggers of KD infection. Recently, bacterial toxins or intracytoplasmic inclusion bodies associated with viral infections have been newly hypothesized as causes for the development of KD18).
In the present study, PCT levels were not significantly different between patients with complete KD and those with incomplete KD. However, more nonresponders than responders had a PCT level >1.0 ng/mL, and there was a significant difference in PCT between responders and nonresponders in the KD group after adjustment for age by using ANCOVA. Another study investigating responders and nonresponders to initial IVIG treatment demonstrated that PCT levels were significantly higher in nonresponders than in responders10). This result was similar to the findings of our study. A recent study enrolled 77 patients with complete KD and 24 patients with incomplete KD, and showed that the PCT levels of patients with complete KD were significantly higher than those of patients with incomplete KD11). The authors described that nonresponders to initial IVIG more frequently had complete KD than incomplete KD (16% vs. 4%). We suspect that more nonresponders with complete KD had higher PCT levels than those with incomplete KD. The PCT level seems to be useful in predicting IVIG response in patients with KD.
Recent guidelines for determining whether antibiotic therapy should be implemented use the following criteria: PCT<0.25 ng/mL, antibiotics strongly discouraged; PCT<0.5 ng/mL, antibiotics discouraged; and PCT>1.0 ng/mL, antibiotics strongly encouraged13). Of the 49 KD patients in the present study, 37 (76%) would be recommended to not receive antibiotics (PCT<0.5 ng/mL). In comparison, 99 patients (89%) with viral infections and 16 patients (67%) with bacterial infections would be recommended to not receive antibiotics. As stated above, PCT levels were significantly different between patients with KD and those with viral infection, and between patients with viral infection and those with bacterial infection, but not between patients with KD and those with bacterial infection. Although PCT levels were not useful in differentiating between KD and bacterial infections, ESR and CPR levels were significantly higher in patients with KD than in those with bacterial infection. Therefore, not only PCT but also ESR and CRP levels should be considered in febrile pediatric patients. If febrile patients have high ESR and CRP levels and low PCT levels in addition to 2 or 3 symptoms of KD, KD should be suspected rather than bacterial infection.
The limitations of the present study were the small sample size, especially patients with incomplete KD and patients with KD who were IVIG nonresponders, and the lack of comparisons with other systemic inflammatory conditions such as autoimmune disease. Further studies on cytokines, including TNF-α and IL-6, will help in determining the role of PCT in KD.
In conclusion, the PCT levels may help differentiate KD from bacterial infections. A combination of disease markers including ESR, CRP, and PCT may be useful for differentiating between KD and viral or bacterial infections. In addition, the PCT level may be somewhat useful in predicting responses to IVIG treatment.
Notes
Conflict of interest:
No potential conflict of interest relevant to this article was reported.
References
2. Simon L, Gauvin F, Amre DK, Saint-Louis P, Lacroix J. Serum procalcitonin and C-reactive protein levels as markers of bacterial infection: a systematic review and meta-analysis. Clin Infect Dis 2004;39:206–217.


3. Delèvaux I, André M, Colombier M, Albuisson E, Meylheuc F, Bègue RJ, et al. Can procalcitonin measurement help in differentiating between bacterial infection and other kinds of inflammatory processes? Ann Rheum Dis 2003;62:337–340.



4. Xu RY, Liu HW, Liu JL, Dong JH. Procalcitonin and C-reactive protein in urinary tract infection diagnosis. BMC Urol 2014;14:45



5. Vouloumanou EK, Plessa E, Karageorgopoulos DE, Mantadakis E, Falagas ME. Serum procalcitonin as a diagnostic marker for neonatal sepsis: a systematic review and meta-analysis. Intensive Care Med 2011;37:747–762.


6. Cies JJ, Chopra A. Procalcitonin use in a pediatric intensive care unit. Pediatr Infect Dis J 2014;33:984–986.


8. Newburger JW, Takahashi M, Gerber MA, Gewitz MH, Tani LY, Burns JC, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Circulation 2004;110:2747–2771.


9. Okada Y, Minakami H, Tomomasa T, Kato M, Inoue Y, Kozawa K, et al. Serum procalcitonin concentration in patients with Kawasaki disease. J Infect 2004;48:199–205.


10. Yoshikawa H, Nomura Y, Masuda K, Koriya C, Arata M, Hazeki D, et al. Serum procalcitonin value is useful for predicting severity of Kawasaki disease. Pediatr Infect Dis J 2012;31:523–525.


11. Cho HJ, Choi YE, Song ES, Cho YK, Ma JS. Procalcitonin levels in patients with complete and incomplete Kawasaki disease. Dis Markers 2013;35:505–511.



12. Newburger JW, Takahashi M, Gerber MA, Gewitz MH, Tani LY, Burns JC, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Pediatrics 2004;114:1708–1733.


13. Bouadma L, Luyt CE, Tubach F, Cracco C, Alvarez A, Schwebel C, et al. Use of procalcitonin to reduce patients' exposure to antibiotics in intensive care units (PRORATA trial): a multicentre randomised controlled trial. Lancet 2010;375:463–474.


14. Nijsten MW, Olinga P, The TH, de Vries EG, Koops HS, Groothuis GM, et al. Procalcitonin behaves as a fast responding acute phase protein in vivo and in vitro. Crit Care Med 2000;28:458–461.


15. Fernández Lopez A, Luaces Cubells C, García García JJ, Fernández Pou J. Spanish Society of Pediatric Emergencies. Procalcitonin in pediatric emergency departments for the early diagnosis of invasive bacterial infections in febrile infants: results of a multicenter study and utility of a rapid qualitative test for this marker. Pediatr Infect Dis J 2003;22:895–903.


16. Esposito S, Tagliabue C, Picciolli I, Semino M, Sabatini C, Consolo S, et al. Procalcitonin measurements for guiding antibiotic treatment in pediatric pneumonia. Respir Med 2011;105:1939–1945.


17. Hatzistilianou M, Rekliti A, Athanassiadou F, Catriu D. Procalcitonin as an early marker of bacterial infection in neutropenic febrile children with acute lymphoblastic leukemia. Inflamm Res 2010;59:339–347.


18. Galeotti C, Bayry J, Kone-Paut I, Kaveri SV. Kawasaki disease: aetiopathogenesis and therapeutic utility of intravenous immunoglobulin. Autoimmun Rev 2010;9:441–448.


Fig. 1
The number of patients with Kawasaki disease (KD) in each procalcitonin (PCT) level group. (A) The proportion of individuals with each PCT level was not significantly different between the 38 complete and 8 incomplete KD patients (P=0.477). (B) The proportion of patients in each PCT level group significantly differed between the 38 responders and the 8 nonresponders (P=0.01). Of the 49 KD patients, 5 who had 0.5<PCT levels<1.0 were excluded in the figure.



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link PubMed
PubMed Download Citation
Download Citation


