< Previous Next >
Article Contents
| Korean J Pediatr > Volume 60(8); 2017 |
|
Abstract
Purpose
The aim of this study was to assess the clinical characteristics of hypertensive encephalopathy according to the underlying etiologies in children.
Methods
We retrospectively evaluated 33 pediatric patients who were diagnosed as having hypertensive encephalopathy in Chonbuk National University Children's Hospital. Among the patients, 18 were excluded because of incomplete data or because brain magnetic resonance imaging (MRI) was not performed. Finally, 17 patients were enrolled and divided into a renal-origin hypertension group and a non-renal-origin hypertension group according to the underlying cause. We compared the clinical features and brain MRI findings between the 2 groups.
Results
The renal group included renal artery stenosis (4), acute poststreptococcal glomerulonephritis (2), lupus nephritis (2), and acute renal failure (1); the nonrenal group included essential hypertension (4), pheochromocytoma (2), thyrotoxicosis (1), and acute promyelocytic leukemia (1). The mean systolic blood pressure of the renal group (172.5±36.9 mmHg) was higher than that of the nonrenal group (137.1±11.1 mmHg, P<0.05). Seizure was the most common neurologic symptom, especially in the renal group (P<0.05). Posterior reversible encephalopathy syndrome (PRES), which is the most typical finding of hypertensive encephalopathy, was found predominantly in the renal group as compared with the nonrenal group (66.6% vs. 12.5%, P<0.05).
In children, hypertension is a rare disease with a prevalence of less than 1%1). Despite this, it is still very important because hypertension in children is highly likely to have a serious underlying illness compared to adult patients2).
Hypertension can cause various complications, with hypertensive encephalopathy a particularly unfavorable prognosis. Hypertensive encephalopathy is a common manifestation of malignant hypertension which is characterized by grade III/IV retinopathy and widespread endothelial damage with uncontrolled hypertension3). It could be one of the first symptoms of secondary hypertension in pediatric patients compared to adults. Such malignant hypertension is more commonly associated with renal hypertension in pediatric patients because of the activation of the renin angiotensin system and increased activity of sympathetic tone4).
Hypertensive encephalopathy is defined as acute brain dysfunction such as severe headache, changes in consciousness, seizure, and retinal hemorrhage induced by sustained severe hypertension5). The etiologies of hypertensive encephalopathy are well identified, but the exact pathophysiology of hypertensive encephalopathy has not been established. However, it can be explained in 2 concepts. First, according to autoregulation breakthrough conception, cerebral arterioles are forced to dilate, leading to vasogenic edema6). Second, in overregulation conception, brain vessel spasm in response to acute hypertension results in cerebral ischemia and cytotoxic edema7). Overregulation conception is thought to be related to the release of humoral vasoconstrictors that are normally increased when hypertension occurs. The latter concept is explained as the cause of the most characteristic posterior reversible encephalopathy syndrome (PRES) lesion. In hypertensive encephalopathy, brain edema can cause stimulation of stretch receptors in the fourth ventricle, which can make the hypertension worse8). These theories suggest that hypertensive encephalopathy can have different characteristics depending on the underlying diseases, because each etiology has a different contributing factor for hypertension.
For this reason, we tried to assess the relationship between the underlying causes of hypertensive encephalopathy and clinical characteristics or brain MRI findings in pediatric patients.
The total number of pediatric patients (younger than 20 years of age) who were admitted to our Pediatric Department with hypertension as the main symptom between 2000 and 2015 was 197.
Hypertension was diagnosed when the systolic or diastolic blood pressure was over the 95% percentile for the relevant age categories. All initial blood pressure was measured from an upper extremity with an appropriate pediatric manual arm cuff. Blood pressure was measured continuously when neurologic symptoms were present at the time of admission.
Hypertensive encephalopathy was diagnosed when patient showed hypertension with more than one neurologic symptom including headache, seizure, dysarthria, motor weakness, or loss of consciousness5).
Patients underwent brain MRI and electroencephalography (EEG) within one day of hospitalization. Patients with PRES lesion on brain MRI and those with other findings including infarction or hemorrhage without PRES lesion on brain MRI were classified into ‘typical MRI’ group and ‘atypical MRI’ group, respectively.
We reviewed 33 patients who showed hypertensive encephalopathy. Among them, 16 patients were additionally excluded because of incomplete data (4 patients), or lack of brain imaging study (8 patients), or neurologic symptoms which already had occurred before hypertension onset (4 patients). Consequently, we enrolled 17 children with hypertensive encephalopathy.
Patients were divided into 2 groups according to their cause, and the clinical characteristics of each group were analyzed. The renal group included renal artery stenosis (4), acute poststreptococcal glomerulonephritis (2), lupus nephritis (2), and acute kidney injury (1). The nonrenal group included essential hypertension (4), pheochromocytoma (2), thyrotoxicosis (1), and acute promyelocytic leukemia (1). Blood pressure, clinical manifestations and prognosis, EEG, and brain MRI findings were compared between the groups.
This study was performed with approval from the Institutional Review Board of Chonbuk National University Research Council.
Statistical analysis was conducted with IBM SPSS ver. 18.0 (IBM Co., Armonk, NY, USA). A Pearson chi-square test was used to compare symptoms and radiologic findings between the groups. For comparing blood pressure and prevalence, a simple correlation analysis and Mann-Whitney U test were used. P<0.05 was considered to be statistically significant.
Nine out of 17 patients were included in the renal origin hypertension group and 8 patients were in the nonrenal origin hypertension group (Table 1). All patients were newly diagnosed with hypertensive encephalopathy and no patients had been diagnosed with underlying disease related to hypertension. Mean age of onset was not significantly different between groups (renal group: nonrenal group=11.7:13.0 years old; male:female=8:9; Table 1). In the renal group, mean systolic and diastolic blood pressure was 172.5±36.9 mmHg and 108.7±18.8 mmHg, respectively. Mean systolic blood pressure of nonrenal group was 137.1±11.1 mmHg and the mean diastolic pressure was 87.1±17.0 mmHg. These results had a statistically significant difference (P<0.05).
Eleven out of seventeen patients manifested seizure, which was the most common symptom in our study group (64.7%). Seizure was more common in the renal group, and there was a significant difference when compared to the occurrence of seizure in the nonrenal group (88.9% vs. 37.5%, P<0.05). Seven patients showed seizure before admission, and 4 patients had seizure after admission. Patients who manifested seizure after admission were measured to have symptoms at an average of 180±48.8 minutes after admission. Among them, only 1 patient had progressed to status epilepticus. Nine out of 11 patients showed generalized tonic clonic seizure; the other 2 patients had tonic seizure. Additionally, other clinical presentations included headache, blurred vision, palpitation, and dysarthria (Table 2). Eight patients manifested headache, and half of them had both headache and seizure. Blurred vision (2 patients), palpitation (2 patients), and dysarthria (1 patient) were also seen, but these symptoms usually occurred together rather than as solitary symptoms. The detailed individual results of the patients are shown in Table 3.
Brain MRI was performed in all 17 patients, of whom 7 patients had typical PRES lesion (41.2%), and 2 had multifocal infarction without PRES lesion (11.8%). In the renal group, with all patients manifesting seizures, every patient showed abnormal MRI findings (100%), and 6 patients had PRES lesion (66.6%). All patients with PRES lesions showed seizures, but PRES lesions were improved after treatment (Fig. 1). On the other hand, 3 out of 8 patients showed abnormal MRI findings (37.5%), and only 1 patient (12.5%) showed a PRES lesion in the nonrenal group (P<0.05). Magnetic resonance angiography (MRA) was done only in 5 patients, but there were no significant findings suggesting cerebral vasoconstriction. EEG was performed in 11 patients. EEG results for 8 patients corresponded with MRI findings (72.7%). However, 3 patients had abnormalities only in EEG findings and they had a normal MRI pattern. These patients all belonged to the nonrenal group, as described in Fig. 2.
Clinical outcomes were not as detrimental as already known7). One patient who was diagnosed with lupus nephritis in renal group died (5.8%) and another patient in nonrenal group had dysarthria (5.8%), but the other patients in both groups completely recovered after antiepileptic and hypertensive treatment. Antihypertensive therapy initiated by bolus, and continuous intravenous drug use such as calcium channel blocker (1–5 µg/kg/min) and beta blocker (0.25–3 mg/kg/hr). After acute phase treatment was done, 4 patients in renal group required angiotensin converting enzyme inhibitor (23.5%) for blood pressure control, but other patients in nonrenal group did not take a medicine.
Hypertension in children is usually related to secondary hypertension, unlike in adults in which primary hypertension is the major cause of hypertension7). Malignant hypertension could lead to end-organ damage, but only 1% of hypertension patients undergo a hypertensive crisis5,9). However, if hypertensive crisis occurs among untreated patients, then the mortality rate is approximately 79%10). Therefore, early detection and evaluation for hypertension is very important for decreasing the mortality rate, and at the same time, attempts to resolve an underlying disease should be made. However, the study of pediatric hypertension, especially malignant hypertension, has been limited owing to a low prevalence and difficulty in obtaining data.
Hypertensive encephalopathy can manifest seizures, intracranial hemorrhage, posterior reversible encephalopathy syndrome, and papilledema. In a narrow spectrum, hypertensive encephalopathy has the same meaning as posterior reversible encephalopathy syndrome, but any symptoms related to hypertension with neurologic impairment have been described as hypertensive encephalopathy in other studies9). In adult studies, headaches are the most common symptom, but other studies in the literature describe seizure as a common symptom in hypertensive encephalopathy11,12). In our study, seizure was the most common symptom and this finding was more prominent in the renal origin hypertension group than in the other group (P<0.05). As seizure is an advanced manifestation of malignant hypertension compared to other neurologic symptoms, this result could mean that the renal origin hypertension group had more severe hypertension than the nonrenal origin group in this study.
Many of the young patients with secondary hypertension had renal disease; hence, evaluation of hypertension in children includes the use of renal ultrasonography, echocardiogram, and laboratory findings13,14). However, a direct correlation between hypertensive encephalopathy and renal disease has not been proven. Unlike other etiologies, hypertension originating from renal disease could cause severe hypertension by complex mechanisms, such as renin angiotensin system activation, fluid overload, activated sympathetic tone, and an electrolyte imbalance15,16). This study showed that the renal origin hypertension group had higher mean blood pressure than the nonrenal origin hypertension. Similarly, seizure and PRES pattern MRI scans were easily observed in the renal group, and this is suggestive that typical hypertensive encephalopathy is more common in the renal group than in the nonrenal group.
The differences in the prevalence of typical findings between the groups were related to 2 major factors. First, higher blood pressure can make hypertensive encephalopathy more likely, and these can give rise to typical radiologic findings. Among our study groups, most of the patients with renal disease did not know they had hypertension before a hypertensive crisis attack. Hypertensive encephalopathy was the first symptom of their disease, so their peak and mean blood pressure could be higher than those in the other group because of untreated hypertension. As mentioned in previous studies, acute onset and high blood pressure were directly related with hypertensive encephalopathy onset; therefore, a difference between the 2 groups existed in this study. The other factor is related to a hypertensive encephalopathy mechanism. In the past, auto-regulatory vasoconstriction in the cerebral vasculature was thought to be the main cause of hypertensive encephalopathy17,18). However, in a recent study, vasodilatation according to acute increases in cerebral blood pressure was presented as a more persuasive idea19). Vasodilatation was mediated by prostaglandin synthesis, but sympathetic innervation was used to protect the affected organ. Therefore, a posterior lesion could be the most prevalent lesion in hypertensive encephalopathy because of less sympathetic innervation than the carotid circulation20). The cerebellum, brain stem, parietooccipital junction, basal ganglia, and frontal lobes are usually not affected by hypertensive encephalopathy owing to the same reasons, and pediatric patients could be more vulnerable than adults because of immature autoregulation21). As for the second factor, hypertension in renal disease is induced not only by activation of the sympathetic tone, but also by volume overloading, and hypertensive encephalopathy could occur easily.
Brain MRI is the most effective diagnostic tool in patients with hypertensive encephalopathy22). Cerebral edema induces dark abnormal signals in T1-weight images and bright abnormal signals in T2-weighted images. In our study, typical MRI findings showed the same results as in previous studies, but some patients presented with abnormal findings only on EEG and not MRI. As EEG has a specificity of 78%–98%, it could be more sensitive than radiologic images to detect early phase abnormalities, which did not appear on other images23). In recent studies, reversible cerebral vasoconstriction syndrome (RCVS) should be considered to newly diagnosed PRES patients24). Considering patient's age and symptoms, there is a little possibility to being diagnosed with RCVS in this study group. But to confirm diagnosis, MRA need to be considered in patients who showed atypical and normal MRI findings.
As the mean blood pressure was higher in the renal group, neurologic symptoms and typical MRI findings were also prominent in this group. However, all patients were included in the study based on peak blood pressure, and those who had no neurologic impairment despite high blood pressure were excluded. This finding could be meaningful in itself. This is a limited study because only 17 patients were suitable according to our criteria. Therefore, we cannot generalize the relationship between hypertensive encephalopathy and underlying diseases. For this, a wider range study with adult patients should be performed. Furthermore, other objective parameters, such as serologic biomarkers, could reveal the exact pathophysiology that could explain the corelation between underlying diseases and the initiation of hypertensive encephalopathy.
In conclusion, there are many underlying diseases that induce neurologic impairment. Therefore, when physicians suspect hypertensive encephalopathy, a differential diagnosis is important for proper treatment. The basic treatment for hypertensive encephalopathy is an antihypertensive drug; however, additional treatment for underlying diseases should be administered. Hence, we studied the characteristics of hypertensive encephalopathy in children who had different causes for hypertension compared to adults. In this study, renal disease was found to be a common cause of typical hypertensive encephalopathy. Therefore, it is critical that physicians should be aware of hypertensive encephalopathy and hypertensive crisis in children with renal origin hypertension.
Notes
Conflict of interest:
No potential conflict of interest relevant to this article was reported.
References
1. Gavrilovici C, Boiculese LV, Brumariu O, Dimitriu AG. Etiology and blood pressure patterns in secondary hypertension in children. Rev Med Chir Soc Med Nat Iasi 2007;111:70–81.

2. Croix B, Feig DI. Childhood hypertension is not a silent disease. Pediatr Nephrol 2006;21:527–532.


3. Immink RV, van den Born BJ, van Montfrans GA, Koopmans RP, Karemaker JM, van Lieshout JJ. Impaired cerebral autoregulation in patients with malignant hypertension. Circulation 2004;110:2241–2245.


4. Klein IH, Ligtenberg G, Oey PL, Koomans HA, Blankestijn PJ. Sympathetic activity is increased in polycystic kidney disease and is associated with hypertension. J Am Soc Nephrol 2001;12:2427–2433.


5. Aggarwal M, Khan IA. Hypertensive crisis: hypertensive emergencies and urgencies. Cardiol Clin 2006;24:135–146.


6. Ahmed ME, Walker JM, Beevers DG, Beevers M. Lack of difference between malignant and accelerated hypertension. Br Med J (Clin Res Ed) 1986;292:235–237.



7. Hu MH, Wang HS, Lin KL, Huang JL, Hsia SH, Chou ML, et al. Clinical experience of childhood hypertensive encephalopathy over an eight year period. Chang Gung Med J 2008;31:153–158.

8. Zheng H, Chen C, Zhang J, Hu Z. Mechanism and therapy of brain edema after intracerebral hemorrhage. Cerebrovasc Dis 2016;42:155–169.


9. Cherney D, Straus S. Management of patients with hypertensive urgencies and emergencies: a systematic review of the literature. J Gen Intern Med 2002;17:937–945.



10. Keith NM, Wagener HP, Barker NW. Some different types of essential hypertension: their course and prognosis. Am J Med Sci 1974;268:336–345.


11. Wright RR, Mathews KD. Hypertensive encephalopathy in childhood. J Child Neurol 1996;11:193–196.


12. O'Hara McCoy H. Posterior reversible encephalopathy syndrome: an emerging clinical entity in adult, pediatric, and obstetric critical care. J Am Acad Nurse Pract 2008;20:100–106.


13. Textor SC. Secondary hypertension: renovascular hypertension. J Am Soc Hypertens 2014;8:943–945.


14. Kapur G, Ahmed M, Pan C, Mitsnefes M, Chiang M, Mattoo TK. Secondary hypertension in overweight and stage 1 hypertensive children: a Midwest Pediatric Nephrology Consortium report. J Clin Hypertens (Greenwich) 2010;12:34–39.


15. Baracco R, Kapur G, Mattoo T, Jain A, Valentini R, Ahmed M, et al. Prediction of primary vs secondary hypertension in children. J Clin Hypertens (Greenwich) 2012;14:316–321.



16. Wirrell EC, Hamiwka LD, Hamiwka LA, Grisaru S, Wei X. Acute glomerulonephritis presenting with PRES: a report of 4 cases. Can J Neurol Sci 2007;34:316–321.


17. Strebel SP, Kindler C, Bissonnette B, Tschalèr G, Deanovic D. The impact of systemic vasoconstrictors on the cerebral circulation of anesthetized patients. Anesthesiology 1998;89:67–72.


18. Paulson OB, Strandgaard S, Edvinsson L. Cerebral autoregulation. Cerebrovasc Brain Metab Rev 1990;2:161–192.

19. Cipolla MJ, Smith J, Kohlmeyer MM, Godfrey JA. SKCa and IKCa Channels, myogenic tone, and vasodilator responses in middle cerebral arteries and parenchymal arterioles: effect of ischemia and reperfusion. Stroke 2009;40:1451–1457.



20. Handa Y, Caner H, Hayashi M, Tamamaki N, Nojyo Y. The distribution pattern of the sympathetic nerve fibers to the cerebral arterial system in rat as revealed by anterograde labeling with WGA-HRP. Exp Brain Res 1990;82:493–498.


21. Hinchey J, Chaves C, Appignani B, Breen J, Pao L, Wang A, et al. A reversible posterior leukoencephalopathy syndrome. N Engl J Med 1996;334:494–500.


22. Hamilton BE, Nesbit GM. Delayed CSF enhancement in posterior reversible encephalopathy syndrome. AJNR Am J Neuroradiol 2008;29:456–457.



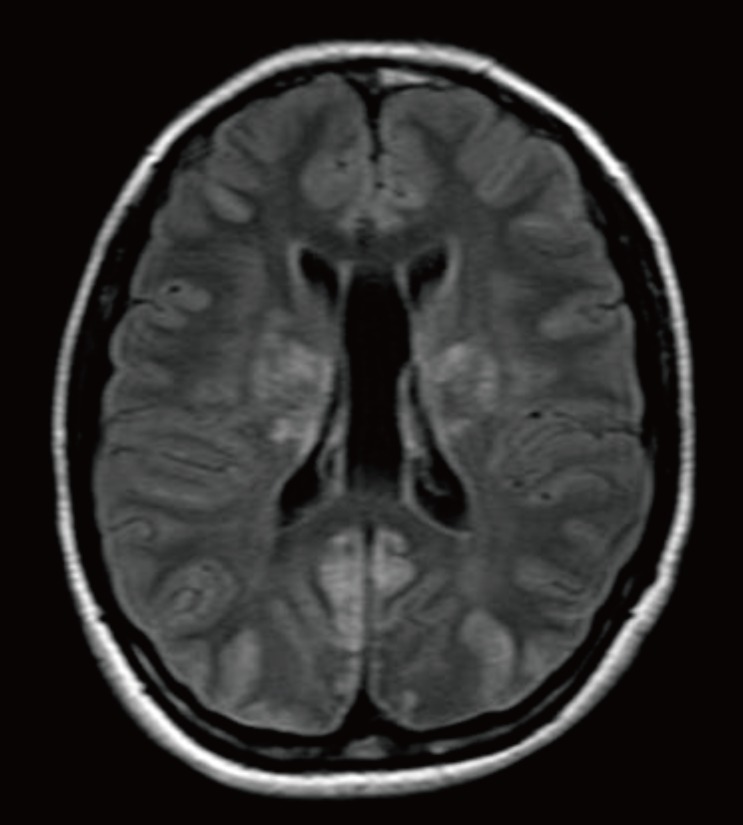
Fig. 1
(A) T2-weighted image of renal-origin hypertensive encephalopathy showing the parieto-occipital lesion, which demonstrates the typical magnetic resonance imaging findings of posterior reversible encephalopathy syndrome (arrow). (B) The 6-month follow-up image showing a full recovery state.

Fig. 2
Nontypical T2-weighted image of renal origin hypertensive encephalopathy, showing high signal intensity on the basal ganglia and left occipital area.



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link PubMed
PubMed Download Citation
Download Citation


