Article Contents
| Korean J Pediatr > Volume 61(1); 2018 |
|
Abstract
Purpose
The aim of this study was to evaluate the diagnostic accuracy of urinary biomarkers, such as neutrophil gelatinase-associated lipocalin (uNGAL) and β-2 microglobulin (uB2MG), in early detection of urinary tract infection (UTI) in infants aged <3 months with fever.
Methods
A total of 422 infants aged <3 months (male:female=267:155; mean age, 56.4 days), who were admitted for fever, were retrospectively included in this study. We compared uNGAL and uB2MG between the UTI and non-UTI groups at the time of admission. The sensitivity, specificity, accuracy, and area under the curve (AUC) of uNGAL and uB2MG for use in diagnosing UTI were assessed.
Results
Among 422 patients, 102 (24.2%) were diagnosed with UTI. Levels of uNGAL were higher in the UTI group than in the non-UTI group (366.6 ng/mL vs. 26.9 ng/mL, P<0.001). Levels of uB2MG were not different between the 2 groups. Multivariate analysis revealed that uNGAL was an independent predictive factor for UTI (P=0.033). The sensitivity, specificity, and accuracy were 90.2%, 92.5%, and 91.9% for uNGAL, and 48.0%, 43.8%, and 44.8% for uB2MG, respectively. AUC of uNGAL was 0.942 and that of uB2MG was 0.407.
Urinary tract infection (UTI) is one of the most common bacterial infections in febrile infants and its prevalence is especially high in infants younger than 3 months. Delayed diagnosis and management of UTI can result in renal damage and loss of renal function.1) Furthermore, infants younger than 3 months with fever had a higher risk of serious bacterial infections, which are difficult to distinguish from simple viral infections because of the vague symptoms.2,3) Therefore, early diagnosis of UTI is important but challenging, especially in infants younger than 3 months with fever.
Although urine culture is the standard approach to confirm the diagnosis of UTI, it takes a long time to obtain the results.4,5) Leukocytosis, neutrophilia, and elevation of erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) are common findings in UTI; however, these are nonspecific markers of inflammation.6) Urinalysis, including urine nitrites, is a helpful screening method to predict UTI; however, it alone may be inadequate due to its nonspecific nature.4,7,8) Based on these findings, it is difficult to diagnose UTI early, especially in febrile infants without an identifiable source. Therefore, it is necessary to find quick, noninvasive, and reliable markers for the early diagnosis of UTI. Recently, to diagnose UTI noninvasively, several studies have reported that some urinary biomarkers of acute kidney injury (AKI) may be associated with UTI.9)
Neutrophil gelatinase-associated lipocalin (NGAL), a 25-kDa protein, is released from the secondary granules of neutrophils and is covalently bound to neutrophil gelatinase. It is a component of the innate immune system against bacterial infections.10,11) NGAL is expressed in various tissues including the kidney. Urinary NGAL (uNGAL) is secreted predominantly by the injured epithelial cells of the kidney.10) It has been identified as one of the earliest and most indicative biomarkers of AKI.10,12,13)
β-2 microglobulin (B2MG), a 11.8-kDa low molecular weight protein, exists in all nucleated cells. It is released into the blood when lymphocytes are activated during inflammation, which is a component of cellular immune response.14) Urinary B2MG (uB2MG) is freely filtered by the glomerulus and most of it is reabsorbed in the proximal tubules, and a part of it is excreted in the urine.14,15) In the presence of a proximal tubule disorder, the level of uB2MG can be elevated. In addition, one study suggested that uB2MG was helpful in differential diagnosis of UTI.15)
A total of 492 patients aged <3 months were admitted to Keimyung University Dongsan Medical Center due to fever (body temperature≥38℃) without a source between January and December 2016, and we retrospectively reviewed the medical records of 422 patients. “fever without a source” was defined when the source of infection was not apparent after examination in a nontoxic infant without significant underlying illness.18)
Of the 492 patients, 70 infants were excluded. Eight patients were excluded because they were born prematurely (<36 weeks of gestation). Eight patients who had previous history of UTI were excluded. Sixteen patients were excluded as they had received antibiotics therapy prior to urine collection. Furthermore, 38 patients were excluded due to the lack of uNGAL and uB2MG records (Fig. 1). There were no patients who had AKI sign such as oliguria or elevation of serum creatinine.
We defined UTI as positive urine culture (single pathogen with ≥100,000 colony-forming units/mL on urine culture). The patients were divided into 2 groups based on the presence or absence of UTI.
The approval of the Institutional Review Board (IRB) was obtained (DSMC 2017-06-015). Informed consent was waived by IRB.
Blood and urine samples were obtained before starting parenteral antibiotics therapy. All blood specimens were collected from the peripheral veins. All urine specimens for urinalysis, estimating the levels of uNGAL, urine creatinine (uCr), uB2MG, and culture were collected in a sterile, adhesive plastic bag after cleaning the perineum to maintain sterile conditions. Urine specimens were analyzed within 1 hour of collection. uCr was determined by the kinetic Jaffe method using an ADVIA2400 chemistry analyzer (Siemens Healthcare Diagnostics Inc., Erlangen, Germany). uNGAL was measured using chemiluminescent microparticle immunoassays in an ARCHITECT i2000SR system (Abbott Diagnostics, Abbott Park, IL, USA). Urinary B2MG was measured with nephelometry using a BNII nephelometer (Dade Behring, Inc., Deerfield, IL, USA; now Siemens Healthcare Diagnostics).
To eliminate the bias that may be introduced by the hydration status, we adjusted the urinary NGAL and B2MG ratio according to the uCr concentration. The grade of pyuria was classified according to the white blood cells (WBCs) count (grade 0, WBC 0–1/high power field [HPF]; grade 1, WBC 2–4/HPF; grade 2, WBC 5–10/HPF; grade 3, WBC 11–20/HPF; grade 4, WBC 20–30/HPF; and grade 5, WBC >30/HPF).
We compared the following clinical and laboratory variables: sex, age, body weight, serum WBC count, serum creatinine, CRP, pyuria grade, urine nitrites, uNGAL, uNGAL/Cr and uB2MG, uB2MG/Cr at the time of admission according to the presence or absence of UTI.
All statistical analyses were performed using IBM SPSS Statistics ver. 22.0 (IBM Co., Armonk, NY, USA). Factors that might be associated with UTI were tested by univariate analysis. A chi-square test was conducted to compare the presence of urine nitrates and the ratio of male to female (M:F) according to UTI. Two sample t tests were used to compare the differences in continuous variables. Then, statistically significant variables were included in the multivariate analysis. Logistic regression analysis was performed to evaluate the effects of different variables. We did not include sex as a covariate in the multivariate analysis to avoid variance inflation given its high correlation with UTI. P values less than 0.05 were considered statistically significant for all tests. The sensitivity, specificity, and accuracy of each variable in diagnosing UTI were calculated using optimal cutoff values based on receiver operating characteristic curve analysis. Efficacies of each variable to predict UTI were measured by the area under the curve (AUC).
We reviewed the data of 492 patients and excluded 70 patients. Among 422 patients (M:F=267:155; mean age, 56.4 days), 102 patients (24.2%) were classified into the UTI group and 320 (75.8%) into the non-UTI group. Escherichia coli was the most common causative bacteria of UTI. The causative bacteria in our study were as follows: E. coli, 91; Klebsiella pneumoniae, 5; Enterococcus faecalis, 3; Streptococcus agalactiae, 2; Staphylococcus aureus, 1.
The UTI group had higher proportion of males than the non-UTI group (85.3% vs. 56.3%, P<0.001). The mean body weight was significantly higher in the UTI group than in the non-UTI group (5.8 kg vs. 5.3 kg, P=0.001). The patients were older in the UTI group compared to those in the non-UTI group, but it was not statistically significant (60.0 days vs. 55.3 days, P=0.087). The mean values of uNGAL and uNGAL/Cr ratio were significantly higher in the UTI group than in the non-UTI group (366.6 ng/mL vs. 26.9 ng/mL, P<0.001 and 22.3 vs. 4.6, P<0.001, respectively). The mean value of uB2MG was higher in the UTI group than in the non-UTI group, but it was not statistically significant (2.9 mg/L vs. 2.8mg/L, P=0.918). uB2MG/Cr ratio in the UTI group was lower than in the non-UTI group (0.1 vs. 0.2, P=0.030). Serum WBC count, CRP, pyuria grade, and positive urine nitrites were significantly higher in the UTI group than in the non UTI group (P<0.001 for each) (Table 1).
Univariate analysis revealed several variables associated with UTI. In univariate analysis, CRP, Pyuria grade, urine nitrite, and urine NGAL were significant. Also, CRP (P=0.010), pyuria grade (P<0.001), urine nitrate (P=0.002), and uNGAL (P=0.033) were statistically significant independent factors to predict UTI in multivariate analysis (Table 2).
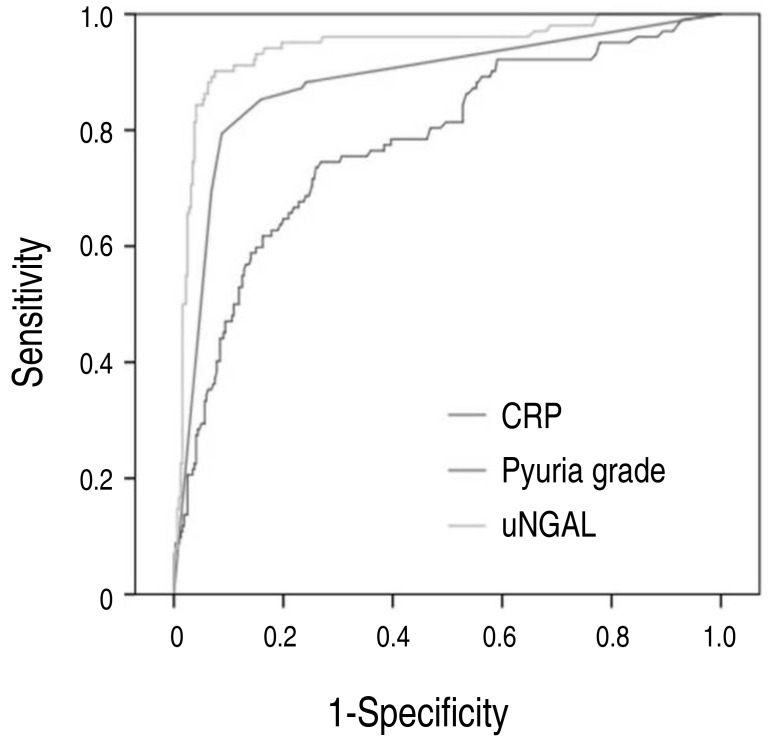
The optimal cutoff value of uNGAL was 46.2 ng/mL, with 90.2% sensitivity, 92.5% specificity, and 91.9% accuracy. The optimal cutoff value of uB2MG was 0.5 mg/L, with 48.0% sensitivity, 43.8% specificity, and 44.8% accuracy. The sensitivity, specificity, and accuracy of CRP were 73.5%, 74.1%, and 73.9% with an optimal cutoff value of 1.61 ng/mL. The sensitivity, specificity and accuracy of pyuria grade were 79.4%, 91.3%, and 88.4% with an optimal cutoff value of grade 4. The sensitivity, specificity, and accuracy of urine nitrite were 48.0%, 43.8%, and 88.4% (Table 3). AUC of uNGAL (0.942) was greater than that of uB2MG (0.407), CRP (0.778), pyuria grade (0.882), and urine nitrite (0.711) (Fig. 2).
UTI is one of the most common bacterial infections especially in febrile infants aged <3 months. These infants have a higher risk of serious bacterial infections and it is more difficult to distinguish severe bacterial infections from simple viral infections because many of these infants appear well and have no localizing signs.2,3) In the present study, we found that the prevalence of culture proven UTI was 24.2% among infants presenting with fever, which is higher than that of a 1999 study conducted by the American Academy of Pediatrics (AAP) in which the prevalence of UTI was 5%19) and that of a study by Shaikh et al.20) in 2008 in which the prevalence was 7% among infants presenting with fever. The incidence of UTIs in our study was higher than other studies, suggesting that children with specific symptoms were already excluded. Also, several studies suggested that obesity was highly associated with UTI.21) Similarly, the body weight was significantly higher in UTI group in our study.
Urine culture, a standard approach to confirm the diagnosis of UTI, takes at least 24 hours to identify UTI.4,5) Furthermore, it is difficult to obtain clean and adequate urine sample for culture. Urinalysis with urine nitrite alone may be an inadequate screening method to predict UTI due to its nonspecific nature.4,7,8) In our study, 12 patients had culture proven UTI without pyuria. There are several reports that certain bacteria such as Proteus may not result in pyuria.22) Urinalysis (positive was defined as >5 WBC/HPF, positive leukocyte esterase, or positive nitrite) was positive in only 69% of samples with positive urine cultures in a retrospective review of children aged 2 months through 2 years presenting with fever.23) Therefore, it is necessary to find an alternative reliable marker for the early diagnosis of UTI in febrile infants.
NGAL is a recently identified biomarker for diagnosing UTI. It has been identified in different human tissues, including renal proximal tubules and neutrophil granules.24) The elevation of NGAL is believed to be caused by the increased release of activated neutrophils to prevent bacterial iron uptake and growth.16) Urine NGAL increased in both, upper and lower UTI, suggesting that this molecule may be involved in various stages of UTI. Urinary NGAL concentrations decline with age in infants.25) This may be, in part, because premature kidneys would still be developing and NGAL levels play an important developmental role in proliferating nephrons.26) We corrected uNGAL values with uCr to reflect hydration status. However, there was a difference between the results of uNGAL and uNGAL/Cr. This may be also related to immature renal function.
Lee et al.24) suggested that uNGAL could be helpful in diagnosing febrile UTI in children. Ichino et al.16) demonstrated that uNGAL may be a potential noninvasive diagnostic biomarker of renal scarring in children. However, Kim et al.27) reported that the value of uNGAL correlates with the presence of leukocytes in the urine samples, but not with UTI. We found uNGAL to be a relatively sensitive (90.2%), specific (92.5%), and accurate (91.9%) test for diagnosing UTI in infants. uNGAL has been demonstrated to be a sensitive and specific early marker of AKI in infants under the age of 3 months. Future studies are required to elucidate which stages of UTI are associated with uNGAL and determine the indications for its use.
Sherman et al.28) reported that B2MG was used as a marker for kidney injury including segmental glomerulosclerosis, pyelonephritis, nephrosclerosis, and minimal change nephropathy. Kim and Lim15) found that 24-hour uB2MG is useful to distinguish acute pyelonephritis from lower UTI in children. Oh et al.29) suggested that spot uB2MG values were correlated with the presence of renal defects in children with UTI. However, Kangari et al.30) reported that uB2MG was not sufficient to be used as a diagnostic marker for predicting renal injury because of lower sensitivity and specificity compared with WBC count, ESR, and CRP in childhood UTI. In our study, uB2MG level was not associated with UTI (P=0.918). There have been several reports suggesting that uB2MG concentrations elevated three folds by day 3 of UTI, whereas NGAL levels increased earlier (12 hours to 3 days).31)
Lin et al.32) reported that sensitivity and specificity of pyuria (≥5 WBCs/HPF) were 59%, 93% for diagnosis of UTI in febrile infants younger than 8 weeks of age. Sensitivity and specificity of CRP (>2 mg/dL) were 59%, 90%, respectively.32) According to 2011 AAP guideline, sensitivity and specificity of the pyuria were 73% (range, 32%–100%), 81% (range, 45%–98%) in febrile UTI infants and children 2 to 24 months.4) We found that uNGAL was more sensitive than CRP and pyuria grade in diagnosing UTI in infants younger than 3 months with fever.
However, there are several limitations in this study. First, since all urine samples were obtained in a urine bag and our UTI criteria did not include pyuria, contamination and overdiagnosis is a concern. Second, we observed only 2 biomarkers. Recently, to diagnose UTI noninvasively, several studies have reported that some urinary biomarkers of AKI may be associated with UTI in children.9) These biomarkers include cystatin C, NGAL, B2MG, kidney injury molecule 1,33) liver-type fatty acid-binding protein, interleukin 18, insulin-like growth factor-binding protein 7, tissue inhibitor of metalloproteinase 2, calprotectin, urine angiotensinogen34) and urine micro-RNA.11) Further studies evaluating many other urinary biomarkers are needed to identify a more sensitive marker in predicting AKI in infants. Third, there was no time-dependent data of urinary biomarkers. It is possible that the selection of time-points account for differences in the ability of these 2 biomarkers in detecting UTI in febrile infants.35) Fourth, since this study was a single center retrospective study, the sample size was small and the age was limited to 3 months. Further prospective multicenter randomized control studies are needed in children of a wider range of age to overcome these limitations.
In conclusion, UTI is the most prevalent cause of fever in infants. When considering the long-term consequences of UTI, early diagnosis and immediate treatment are important. Since the diagnostic accuracy of uNGAL is high, it can help in the early diagnosis and treatment of UTI in febrile infants <3 months of age with nonspecific symptoms.
Notes
Conflict of interest:
No potential conflict of interest relevant to this article was reported.
References
1. Desai DJ, Gilbert B, McBride CA. Paediatric urinary tract infections: Diagnosis and treatment. Aust Fam Physician 2016;45:558–563.

2. Luszczak M. Evaluation and management of infants and young children with fever. Am Fam Physician 2001;64:1219–1226.

3. Bachur RG, Harper MB. Predictive model for serious bacterial infections among infants younger than 3 months of age. Pediatrics 2001;108:311–316.


4. Subcommittee on Urinary Tract Infection, Steering Committee on Quality Improvement and Management. Roberts KB. Urinary tract infection: clinical practice guideline for the diagnosis and management of the initial UTI in febrile infants and children 2 to 24 months. Pediatrics 2011;128:595–610.


5. Lee H, Kim KB, Lee S, Lee SW, Kim M, Cho SY, et al. Urodynamic assessment of bladder and urethral function among men with lower urinary tract symptoms after radical prostatectomy: a comparison between men with and without urinary incontinence. Korean J Urol 2015;56:803–810.



6. Elder JS. Urinary tract infection. Kliegman RM, Stanton BF, St. Geme JW III, Schor NF, Behrman RE, editors. Nelson textbook of pediatrics. 20th ed. Philadelphia (PA): Elsevier, 2016;:2556–2562.
7. Choi DM, Heo TH, Yim HE, Yoo KH. Evaluation of new American Academy of Pediatrics guideline for febrile urinary tract infection. Korean J Pediatr 2015;58:341–346.



8. Shaikh N, Shope TR, Hoberman A, Vigliotti A, Kurs-Lasky M, Martin JM. Association between uropathogen and pyuria. Pediatrics 2016;138.
10. Devarajan P. Neutrophil gelatinase-associated lipocalin: a promising biomarker for human acute kidney injury. Biomark Med 2010;4:265–280.



11. Kashani K, Cheungpasitporn W, Ronco C. Biomarkers of acute kidney injury: the pathway from discovery to clinical adoption. Clin Chem Lab Med 2017;55:1074–1089.


12. Seibert FS, Pagonas N, Arndt R, Heller F, Dragun D, Persson P, et al. Calprotectin and neutrophil gelatinase-associated lipocalin in the differentiation of pre-renal and intrinsic acute kidney injury. Acta Physiol (Oxf) 2013;207:700–708.


13. Wheeler DS, Devarajan P, Ma Q, Harmon K, Monaco M, Cvijanovich N, et al. Serum neutrophil gelatinase-associated lipocalin (NGAL) as a marker of acute kidney injury in critically ill children with septic shock. Crit Care Med 2008;36:1297–1303.



14. Al-Taee IK, Al-Safar JJ, Al-Falahi YS, Al-Shamma IA. The clinical significance of beta2-microglobulin in end-stage renal disease. Saudi J Kidney Dis Transpl 2003;14:492–496.

15. Kim DW, Lim IS. Analysis of urine β2-microglobulin in pediatric renal disease. Korean J Pediatr 2007;50:369–375.

16. Ichino M, Kusaka M, Kuroyanagi Y, Mori T, Morooka M, Sasaki H, et al. Urinary neutrophil-gelatinase associated lipocalin is a potential noninvasive marker for renal scarring in patients with vesicoureteral reflux. J Urol 2010;183:2001–2007.


17. Tullus K. Difficulties in diagnosing urinary tract infections in small children. Pediatr Nephrol 2011;26:1923–1926.


18. Baraff LJ. Management of fever without source in infants and children. Ann Emerg Med 2000;36:602–614.


19. Downs SM. The Urinary Tract Subcommittee of the American Academy of Pediatrics Committee on Quality Improvement. Technical report: urinary tract infections in febrile infants and young children. Pediatrics 1999;103:e54.


20. Shaikh N, Morone NE, Bost JE, Farrell MH. Prevalence of urinary tract infection in childhood: a meta-analysis. Pediatr Infect Dis J 2008;27:302–308.


21. Byun HJ, Ha JY, Jung W, Kim BH, Park CH, Kim CI. The impact of obesity on febrile urinary tract infection and renal scarring in children with vesicoureteral reflux. J Pediatr Urol 2017;13:6767.e1–67.e6.
23. Waseem M, Chen J, Paudel G, Sharma N, Castillo M, Ain Y, et al. Can a simple urinalysis predict the causative agent and the antibiotic sensitivities? Pediatr Emerg Care 2014;30:244–247.



24. Lee HE, Kim DK, Kang HK, Park K. The diagnosis of febrile urinary tract infection in children may be facilitated by urinary biomarkers. Pediatr Nephrol 2015;30:123–130.


25. Zwiers AJ, de Wildt SN, de Rijke YB, Willemsen SP, Abdullahi NS, Tibboel D, et al. Reference intervals for renal injury biomarkers neutrophil gelatinase-associated lipocalin and kidney injury molecule-1 in young infants. Clin Chem Lab Med 2015;53:1279–1289.

26. Lavery AP, Meinzen-Derr JK, Anderson E, Ma Q, Bennett MR, Devarajan P, et al. Urinary NGAL in premature infants. Pediatr Res 2008;64:423–428.


27. Kim BH, Yu N, Kim HR, Yun KW, Lim IS, Kim TH, et al. Evaluation of the optimal neutrophil gelatinase-associated lipocalin value as a screening biomarker for urinary tract infections in children. Ann Lab Med 2014;34:354–359.



28. Sherman RL, Drayer DE, Leyland-Jones BR, Reidenberg MM. N-acetyl-beta-glucosaminidase and beta 2-microglobulin. Their urinary excretion in patients with renal parenchymal disease. Arch Intern Med 1983;143:1183–1185.


29. Oh HS, Bae SP, Kim SS, Park KB. Usefulness of spot urine β2-microglobulin in pediatric acute pyelonephritis. Soonchunhyang Med Sci 2012;18:91–94.

30. Kangari G, Esteghamati M, Ghasemi K, Mahboobi H. Predictive accuracy of urinary beta2-microglobulin for kidney injury in children with acute pyelonephritis. Iran J Kidney Dis 2015;9:19–24.

31. George B, Wen X, Mercke N, Gomez M, O'Bryant C, Bowles DW, et al. Profiling of kidney injury biomarkers in patients receiving cisplatin: time-dependent changes in the absence of clinical nephrotoxicity. Clin Pharmacol Ther 2017;101:510–518.



32. Lin DS, Huang SH, Lin CC, Tung YC, Huang TT, Chiu NC, et al. Urinary tract infection in febrile infants younger than eight weeks of Age. Pediatrics 2000;105:E20.


33. Westhoff JH, Seibert FS, Waldherr S, Bauer F, Tönshoff B, Fichtner A, et al. Urinary calprotectin, kidney injury molecule-1, and neutrophil gelatinase-associated lipocalin for the prediction of adverse outcome in pediatric acute kidney injury. Eur J Pediatr 2017;176:745–755.


Fig. 1
Study flow diagram. UTI, urinary tract infection; uNGAL, urinary neutrophil gelatinase-associated lipocalin; uB2MG, urinary β-2 microglobulin.

Fig. 2
Receiver operating characteristic curve analysis of biomarkers in diagnosis of urinary tract infection. CRP, C-reactive protein; uNGAL, urinary neutrophil gelatinase-associated lipocalin.




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link PubMed
PubMed Download Citation
Download Citation


