Article Contents
| Korean J Pediatr > Volume 59(Suppl 1); 2016 |
|
Abstract
Wilms' tumor is the most common malignant renal tumor in childhood. The brain metastasis of a Wilms' tumor with anaplastic histopathology is rare. We present the case of an 8-year-old girl with Wilms' tumor, who presented with multiple brain metastases 5 years after her primary diagnosis. The brain masses were diagnosed after a generalized tonic-clonic seizure attack. The big solid mass in the cerebellum was resected, and whole-brain radiotherapy was performed, after which, she succumbed to her disease. In the case of clinical suspicion, cranial surveillance should be included in the routine clinical work-up for Wilms' tumor. Combined aggressive therapy (surgery+radiotherapy+chemotherapy) should be applied whenever possible, for both better survival and palliative aspects.
Wilms' tumor is the second most common solid neoplasm and the most common malignant renal tumor in childhood affecting 1/1,000 of children1,2). Survival correlates with tumor histology, as children, with favorable tumor histology have good survival rates despite widespread disease (5-year survival rate is over 80%)3). Lung is the most common metastatic site thereafter liver, lymph nodes and bone2). We present a rare case of Wilms' tumor with multiple brain metastases harboring anaplastic features.
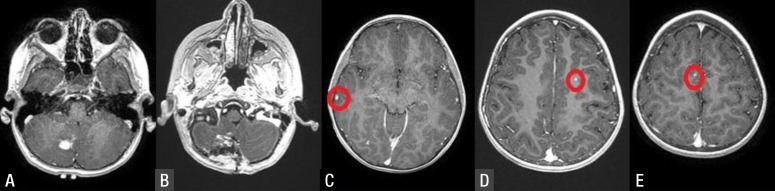
An 8-year-old girl, with a previous diagnosis of Wilms' tumor, admitted to our Emergency Department with new onset generalized tonic-clonic seizure. After we stabilized her status, emergency computed tomography scan showed posterior fossa intraparenchymal mass. Contrast enhanced brain magnetic resonance imaging depicted homogenously enhanced multiple small metastases except a larger one in the right cerebellar parenchyma (Fig. 1).
Her primary illness had been diagnosed 5 years ago. She had had masses in both kidneys and had had distant metastases to lungs at the time of diagnosis (stage V). She had been treated with ifosfamide-carboplatin-etoposide regimen. She had been operated for bilateral kidney masses 3 months after her chemotherapy and had been put on chemotherapy once again after the surgery. She had had a surgery for her lung 1 year ago. She had been put on routine follow-ups since then. In her last clinic visit, distant metastases in the brain were diagnosed after a seizure attack.
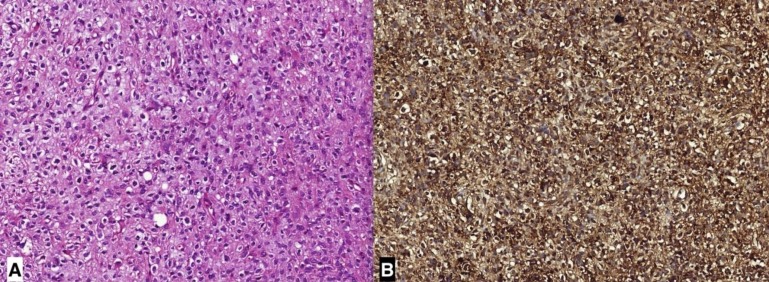
The cerebellar mass was excised via craniotomy. The tumor mass was sharply demarcated from the normal cerebellar tissue. The tumor had immature mesenchymal cells. Stromal component had both spindle and cartilaginous appearance. The cells were giant-multinucleated, atypical and bizarre. There was diffuse anaplasia with atypical mitotic figures. In immunohistochemical staining, WT1 and GFAP were positive; CK was negative (Fig. 2). After craniotomy, whole brain radiotherapy was given in another clinic, however her cranial masses progressed in time and she succumbed to her disease.
Brain metastasis of Wilms' tumor is very rare (1%–2%)1). The patient described in this case report, had been diagnosed of Wilms' tumor 5 years ago and had been treated accordingly. Her brain metastases were diagnosed after a first-time tonic-clonic seizure attack.
While recurrence of Wilms' tumor has been reported up to 25 years after primary diagnosis4), brain metastases usually appear in 2 years. Malogolowkin et al.5) made a meta-analysis of 10 consecutive trials conducted in Wilms' tumor patients. They defined ‘late recurrences’ as recurrences appearing 5 years after initial diagnosis. A total of 13,330 patients were enrolled into the analysis, which had a late recurrence rate of 0.5%. Median time to late recurrence was 13.2 years and mortality rate was 50%. Twenty percent of late recurrences occurred in stage V patients. Three out of 70 patients with late recurrences had anaplastic histology. Abdomen, lungs and contralateral kidney were found to be the most common sites of recurrences in these patients. Three or more medications had been used in 32% and radiotherapy had been used in 37% of the patients with late recurrences5). A huge difference in survival rates was present between the patients with recurrences in contralateral kidney and patients with distant organ recurrences (87% vs. 45%) due to different cell lines they harbor. This means that recurrence in the contralateral kidney might not be a true metastasis, rather a de novo disease5).
Abnormalities in Wilms' tumor suppressor genes Wt-1 (on 11p13) and Wt-2 (on 11p15) have been notified in the literature6). Immunohistochemical studies in the presented case were positive for Wt-1. Beside these tumor suppressor genes, there are many defined molecular abnormalities on chromosomes 1p, 7p, 16q, 17p, and 19q7,8). Late recurrent diseases might have pathologic properties different from the primary disease4). Our patient had been treated in an outside clinic previously and we could not retrieve the past pathology slides to make a comparison between initial and late recurrent disease.
In recent practice, solid tumor resection and adjuvant radiotherapy with chemotherapies are treatment of choices with good results. There is conflicting data about treatment approach to late recurrences of Wilms' tumor. While some authors insist on aggressive therapy, some others recommend to treat these tumors same as recurrent disease in general5,9). There is also no consensus over treatment modality for brain metastases of Wilms' tumor. However, median survival is 1 month for untreated patients, and sole treatment with only surgery or only radiotherapy is not enough for a long survival time2,10,11,12,13). Whole (30 Gy in 10 fractions) or local radiotherapy (30–36 Gy in 1.5–1.8 Gy fractions) to brain metastases are method of choices. A treatment regimen of vincristine, actinomycin D and cyclophosphamide were being used conveniently, however ifosfamide and etoposide have turned to be superior in terms of treatment success2). The patient we presented had one solid mass in the cerebellum and multiple small metastases in the whole brain. We resected the solid tumor totally, and gave adjuvant radiotherapy to the whole brain, yet the patient died after the radiotherapy. One other point to consider in our patient was bilateral kidney masses at the initial presentation. Turkish Pediatric Oncology Group has recommended preoperative chemotherapy regimen in bilateral kidney disease. After initial chemotherapy, the patient had had a nephron sparing surgery in both kidneys. Bilateral nephron sparing surgery is a challenging situation against aggressive chemotherapy regimen in respect of toxicity14).
Prognostic factors for patients with relapse are site of relapse, length of initial remission, initial treatment regimen (2 or 3 drugs), anaplasia and whether initially treated on National Wilms' Tumor Studies 2 or 315). Histologic pattern is important and anaplasia correlates with biologic aggressiveness. Despite surgery and whole brain radiotherapy masses in our patient progressed.
When there is clinical suspicion, cranial surveillance should be added to routine clinical work-up of Wilms' tumor follow-up to detect intracranial masses in a timely manner. Treatment should be conceptualized according to the patient. Combined aggressive therapy (surgery+radiotherapy+chemotherapy) should be applied whenever it is possible for both better survival and palliative aspects.
Acknowledgments
Murat Şakir Ekşi was supported with a postdoctoral fellowship grant by Tubitak (The Scientific and Technological Research Council of Turkey) (grant number: 1059B191400255).
Notes
Conflict of interest:
No potential conflict of interest relevant to this article was reported.
References
1. Harada K, Nishizaki T, Kwak T, Fujisawa H, Nishikawa M, Ito H. Intracranial metastasis of Wilms' tumor involving the tectal plate without pulmonary involvement. Case report. Pediatr Neurosurg 1999;30:331–334.


2. MacRae R, Grimard L, Hsu E, Nizalik E, Halton JM. Brain metastases in Wilms' tumor: case report and literature review. J Pediatr Hematol Oncol 2002;24:149–153.


3. Wilms' tumor: status report, 1990. By the National Wilms' Tumor Study Committee. J Clin Oncol 1991;9:877–887.


4. Lee SY, Kim KR, Park JY, Ro JY. Wilms' tumor with long-delayed recurrence: 25 years after initial treatment. Korean J Urol 2012;53:288–292.



5. Malogolowkin M, Spreafico F, Dome JS, van Tinteren H, Pritchard-Jones K, van den Heuvel-Eibrink MM, et al. Incidence and outcomes of patients with late recurrence of Wilms' tumor. Pediatr Blood Cancer 2013;60:1612–1615.


6. Coppes MJ, Pritchard-Jones K. Principles of Wilms' tumor biology. Urol Clin North Am 2000;27:423–433.


7. Grundy PE, Telzerow PE, Breslow N, Moksness J, Huff V, Paterson MC. Loss of heterozygosity for chromosomes 16q and 1p in Wilms' tumors predicts an adverse outcome. Cancer Res 1994;54:2331–2333.

8. Brown E, Hebra A, Jenrette J, Hudspeth M. Successful treatment of late, recurrent wilms tumor with high-dose chemotherapy and autologous stem cell rescue in third complete response. J Pediatr Hematol Oncol 2010;32:e241–e243.


9. Buyukpamukçu M, Koksal Y, Varan A, Atahan L, Caglar M, Akyuz C, et al. Late recurrence in children with Wilms' tumor. Turk J Pediatr 2007;49:226–230.

10. Lowis SP, Foot A, Gerrard MP, Charles A, Imeson J, Middleton H, et al. Central nervous system metastasis in Wilms' tumor: a review of three consecutive United Kingdom trials. Cancer 1998;83:2023–2029.


11. Sty JR, Starshak RJ, Thorp SM. The role of brain scintigraphy in metastatic Wilms' tumor. Wis Med J 1980;79:28–30.
13. Deutsch M, Albo V, Wollman MR. Radiotherapy for cerebral metastases in children. Int J Radiat Oncol Biol Phys 1982;8:1441–1446.





 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link PubMed
PubMed Download Citation
Download Citation


