Graphical abstract
Prepregnancy body mass index (pre-BMI) is a known factor associated with manageable lifestyle habits that affects the mother’s prognosis as well as fetal and neonatal growth and development [1]. An abnormal pre-BMI contributes to a high risk of negative effects on the mother and the newborn [1]. A high pre-BMI or excessive gestational weight gain (GWG) increases the risk of preeclampsia, gestational hypertension, gestational diabetes, stillbirth, preterm birth, macrosomia, and large for gestational age (LGA) [2]. Conversely, a low pre-BMI or insufficient GWG increases the risk of preterm birth, low birth weight (LBW), and small for gestational age (SGA) [2]. As such, the fetal origins of adult disease hypothesis, which has become widely known, postulates that growth restriction or overweight due to inadequate nutrition is associated with chronic adult diseases such as type 2 diabetes, chronic hypertension, cardiovascular diseases, stroke, and neurocognitive disorders [3]. The well-being of the mother and fetus is directly connected, and the mother’s chronic diseases, smoking, drugs, addiction, uterine problems, and nutritional status affect the fetus [4]. Among them, pre-BMI and GWG reflect maternal nutritional status before and during pregnancy and can predict maternal and infant prognosis [5].
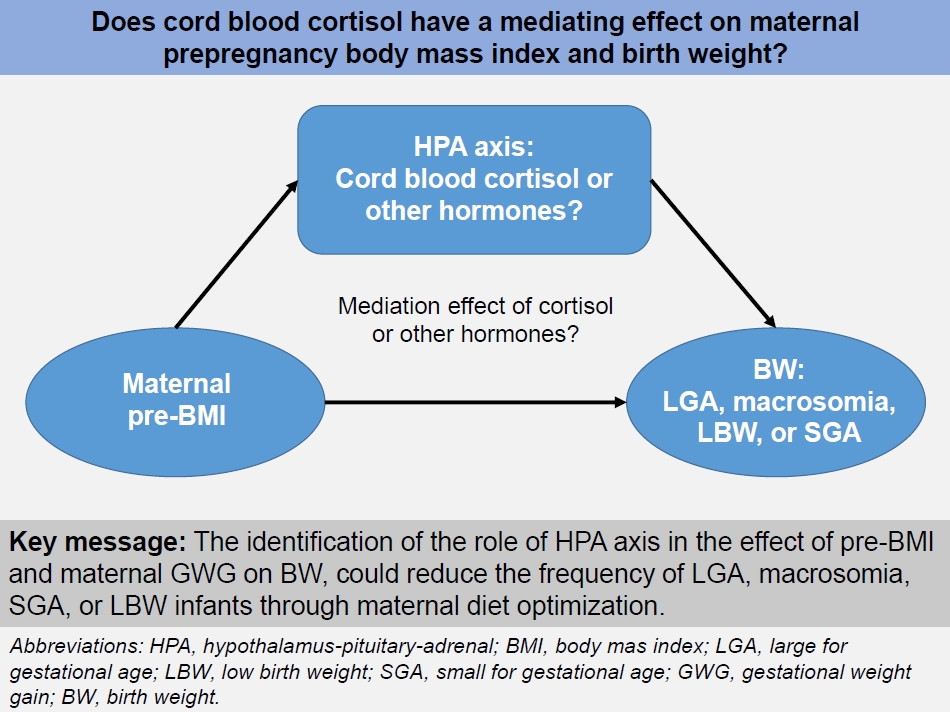
However, the exact mechanism by which pre-BMI and GWG affect birth weight in newborns have not yet been clearly identified. Inappropriate pre-BMI and GWG can affect the maternal or fetal hypothalamic-pituitary-adrenal (HPA) axis, pancreatic cells, and adipose tissue, changing fetal metabolic programming, affecting fetal growth, and influencing neonatal birth weight. While obese mothers in early pregnancy are known to have lower morning cortisol levels than those with a normal BMI, epidemiological and clinical studies have shown that hypercortisolemia is associated with inhibition of fetal development and LBW [6]. Thus, the possibility has been raised that maternal or fetal blood cortisol levels may mediate the relationship between maternal pre-BMI or GWG and birth weight. Therefore, Selvam et al. [7] conducted a hospital-based cross-sectional study to investigate whether cord blood cortisol mediates pre-BMI or GWG and birth weight after assuming cortisol as a hormone representing the HPA axis. The authors reported that the mediation effect of cord blood cortisol on maternal pre-BMI or GWG and birth weight was not statistically significant [7]. Jin et al. [8] analyzed all steroid hormones in the maternal-placental-fetal unit and found that corticosterone had a partial mediating effect of 3.48% and cortisol and corticosterone had a mediating effect of 4.33% and 5.38%, respectively, when the GWG was higher than the Institute of Medicine’s recommendation. Therefore, if the basic mechanisms connecting pre-BMI or GWG and birth weight are identified, achieving an optimal pre-BMI and GWG may reduce obstetric complications as well as the proportion of LGA, macrosomia, SGA, or LBW.
The study of lipid profiles in newborns with LGA and macrosomia or SGA and LBW at birth is very important because it is associated with metabolic syndrome in adulthood [9,10]. However, few studies have examined the change in cord blood lipid profile and maternal pre-BMI, birth weight, and cord blood cortisol. Therefore, Selvam et al. [7] investigated changes in the cord blood lipid profile according to maternal pre-BMI, birth weight, and cord blood cortisol as secondary outcomes. In this study, cord blood high-density lipoprotein cholesterol (HDL-C) levels were negatively correlated with both pre-BMI and birth weight and positively correlated with cord blood cortisol. Pac-Kożuchowska et al. [10] also showed negative correlations between pre-BMI and umbilical cord blood HDL-C in healthy newborns, whereas Mandraha et al. [9] reported no statistically significant differences. In contrast, Mandraha et al. [9] reported that LGA, SGA, or LBW neonates showed statistically significant higher levels of lowdensity lipoprotein cholesterol, very-low-density lipoprotein cholesterol, triglyceride, and cholesterol except HDL-C than appropriate for gestational age neonates. Although Selvam et al. [7] reported that cortisol levels decrease due to maternal and fetal HPA axis dysfunction and maternal obesity and HDL-C concentrations decrease due to HPA axis dysregulation, further animal studies and large-scale multi-center studies are needed to support this claim due to inconsistent results of previous studies.
In conclusion, the exact mechanisms of the fetal origins of adult diseases remain unknown. The ultimate goal is to decrease the frequency of chronic adult diseases by reducing LGA, macrosomia, SGA, and LBW through maternal weight control before and during pregnancy.





 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link PubMed
PubMed Download Citation
Download Citation


