Article Contents
| Clin Exp Pediatr > Volume 66(1); 2023 |
|
Abstract
Background
Myelomeningocele is a lifelong condition that features several comorbidities, such as hydrocephalus, scoliosis, club foot, and lower limb sensory and motor disabilities. Its management has progressed over time, ranging from supportive care to early postnatal closure to prenatal closure of the defect. Recent research discovered that fetal myelomeningocele closure (fMMC) provided superior neurological outcomes to those of postnatal closure. When performed at 12 months of age, fMMC can avert or delay the need for a ventriculoperitoneal shunt and reversed the hindbrain herniation. Moreover, fMMC reportedly enhanced motor function and mental development at 30 months of age. However, its long-term outcomes remain dubious.
Purpose
This systematic review aimed to determine the long-term neurological cognitive, behavioral, functional, and quality of life (QoL) outcomes after fMMC.
Methods
The PubMed, Directory of Open Access Journals, EBSCO, and Cochrane databases were extensively searched for articles published in 2007–2022. Meta-analyses, clinical trials, and randomized controlled trials with at least 5 years of follow-up were given priority.
Graphical abstract.Summary of the review of effects of fetal myelomeningocele closure on cognitive, behavioral, and quality of life outcomes.
Myelomeningocele, the most frequent type of spina bifida, is an inborn malformation of the neural tube that progressively worse with gestational age because the neural tube didn't completely close during early development [1,2]. According to the Centers for Disease Control, myelomeningocele currently affects 3 out of every 10,000 live births, and every year in the United States, 1,500 children are born with spina bifida. Prior to the American government's recommendations in 1992 and 1996 to include folic acid supplements in women's diets, the prevalence was substantially higher [3].
Management for children with myelomeningocele has progressed through time, from supportive care to early postnatal closure to prenatal closure. Recent research discovered that fetal myelomeningocele closure (fMMC) provided superior neurological outcomes than postnatal closure [4]. While myelomeningocele is an embryologic issue, neurologic impairment is also caused by progressive in-utero harm to the exposed spinal cord. In animal study, model of midgestational spinal defect coverage that underwent fMMC showed near normal neurologic function. Early clinical trials reveal that fMMC can correct hindbrain herniation, restore neurologic function, and lessen the need for ventriculoperitoneal shunting [5,6].
However, research on the long-term outcomes of fMMC is still limited [7]. In order to explore the long-term neurological, cognitive, behavioral, functional, and quality of life (QoL) outcomes of individuals with myelomeningocele who received fMMC, this study will comprehensively review the long-term research on fMMC, including big and small studies.
Preferred Reporting Items for Systematic Review and Metaanalysis (PRISMA) protocols were employed in this study. Eligibility criteria were set using the Patient, Intervention, Comparison, and Outcome (PICO) approach. Table 1 shows the PICO criteria.
The databases of PubMed, Directory of Open Access Journals, EBSCO, and Cochrane were extensively researched. The following keywords were used to carry out additional research: “fetal” or “prenatal” or “postnatal” or “myelomeningocele” or “closure” or “surgery” or “long” or “term” or “neurological” or “cognitive” or “behavioral” or “functional” or "quality of life” or “outcome.” Literature searching was conducted along with the librarian of Universitas Sumatera Utara, Medan, Indonesia. The date of publication within 2007–2022 and all studies with more than 5 years of follow-up were the inclusion criteria. All animal studies and systematic reviews were excluded. Microsoft Excel was used to collect the papers.
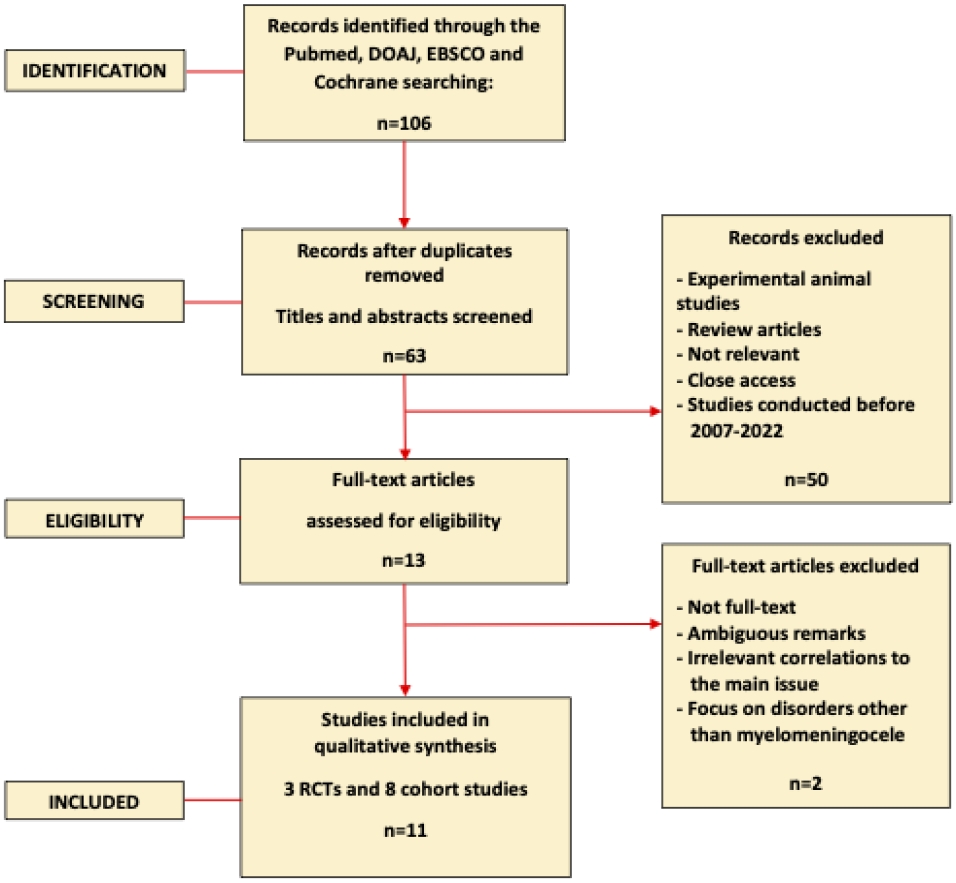
The PRISMA statement indicates that the titles and abstracts of the records that were acquired were checked, and the whole texts of those that were considered relevant were evaluated. Full-text research with meta-analyses, clinical trials, and randomized controlled trials (RCTs) were given priority; thus, a total of 13 research was selected. In the end, 11 research were included in this systematic review. While the other 2 publications were disregarded because of their overlap with other works, their confusing remarks, their irrelevant connections to the core problem, or their focus on diseases other than myelomeningocele. The PRISMA flow diagram for the research identification and selection process is shown in Fig. 1, and the list of publications that made up the systematic review is shown in Table 2.
The primary outcomes of this study were the long-term neurological cognitive, behavioral, and functional outcomes. The secondary outcome was the long-term QoL outcome.
Cochrane Risk for Bias for Randomized Trial was used to assessed bias of every RCTs included in this review. Based on this bias analysis, studies were grouped into 3 groups, i.e., low risk, some concerns, and high risk. For every non-RCTs studies, Cochrane Risk of Bias in Non-Randomized Studies of Interventions was used and all studies were classified into 4 groups, i.e., low, moderate, serious, and critical risk of bias.
Using the initial search strategy, 106 studies were found in total. 43 articles were removed due to the irrelevant title. There were 63 articles left with a relevant title. We eliminated 50 articles based on title and abstract screening. Experimental animal research and literature review articles were excluded. We eventually have 11 articles included in this study after screening and qualitative evaluation. Neurological cognitive, behavioral, functional, and QoL outcomes were the main long-term outcomes discussed among all research.
At 5 years of age, the mean verbal intelligence quotient (VIQ), performance intelligence quotient (PIQ), and full intelligence quotient (FIQ), and processing speed, high-average or average scores of the preschool neurodevelopmental outcome of children following fMMC were reported in 93%, 90%, 90%, and 60%; and were revealed to be within the normal population range by Danzer et al. [8] Individuals who did not require shunt insertion had substantially higher mean VIQ, PIQ, FIQ and processing speeds.
Another research by Danzer et al. [9] examined the preschool functional status following fMMC and showed that most fMMC children had attained cognitive and movement independence but still needed a lot of help with self-care. At 5 years of age, the mean functional quotients for cognitive ability, self-care, mobility, and overall, in fMMC individuals were all considerably lower than age-appropriate population norms. Full caregiver independence was attained by 84%, 38%, 62%, and 58% of fMMC individuals in the areas of cognition, self-care, mobility, and total functional outcome. Individuals with fMMC who were not shunted exhibited greater cognitive, mobility, and overall independence than those who were, as did those with average neurodevelopmental scores. Self-care independence was more prevalent in the nonshunted group and in fMMC individuals with normal neurodevelopmental outcomes [9].
Preterm delivery and fMMC have not been linked to an increase in behavioral issues, impaired social interactions, or restricted behavior patterns in preschool-aged (>5 years) children, according to another research by Danzer et al. [10] For internalizing, externalizing, and overall behavioral issues, the prevalence of "at-risk" or "clinically substantial" scores was comparable to population norms. Between nonshunted and shunted fMMC individuals, there was no difference in the overall internalizing, externalizing, or total behavioral scores. The prevalence of anxiety and depressive, pervasive developmental, and withdrawn behavior was higher in shunted fMMC individuals. There were no indications of oppositional defiance, attention deficit disorder, or hyperactivity [10].
There was no discernible difference in overall adaptive behavior across the surgical groups, according to the study of Houtrow et al. [11] on fMMC and school-age (5.9–10.3 years) functional outcomes. The long-term advantages of fMMC included greater mobility and independence as well as fewer procedures for shunt insertion and correction, but there was no clear evidence of enhanced cognitive functioning. There was no difference in the Vineland composite score. Children in the fMMC group had higher mean percentage scores on the Functional Rehabilitation Evaluation of Sensory-Neurologic Outcomes, lower rates of hindbrain herniation, fewer shunts placed for hydrocephalus, and, among those with shunts, fewer shunt revisions than those in the postnatal group. They also walked without orthotics or assistive devices more frequently [11].
Executive functioning (EF), behavioral adaptive skill (BAS), and long-term neurofunctional results following fMMC were all studied by Danzer et al. [12] fMMC surgery was revealed to enhance long-term functional outcomes. 79% of individuals are community ambulators, 9% are household ambulators, and 14% are wheelchair-dependent at the median follow-up age of 10 years. In contrast, the requirement for tethered cord surgery was connected to a sustained decline in ambulatory status. 26% of individuals had normal bladder function. The majority of individuals who underwent fMMC had average or above-average scores on the Behavioral Regulation Index, Metacognition Index, and Global Executive Composite indices; nonetheless, substantially more of them than the population as a whole exhibited EF deficits in all 3 BRIEF indices. Following fMMC, the general adaptive composite scores tended to perform below average. Normal EF and BAS were predicted by normal early neurodevelopmental outcomes. A considerable BAS impairment was linked to the need for shunting. The majority of fMMC individuals are capable of accomplishing routine jobs at home and school. fMMC enhances long-term ambulatory status. Functional loss is linked to symptomatic spinal cord tethering, whether or not there is an intradural inclusion cyst. The fMMC individuals are more continent than predicted, but bowel and bladder control remain problematic for the fMMC individuals [12].
The physical functional advantages of fMMC for myelomeningocele documented at age 30 months continued throughout school age, according to studies by Houtrow et al. [13] on fMMC and physical functioning. According to the Modified Hoffer Classification, those in the fMMC group tended to be community ambulators and had better self-care skills and gait quality, performed the 10-m walk test 1 second faster, and were able to perform higher-level mobility skills. The likelihood that a child's motor function level would be poorer than their anatomic lesion level was lower in the fMMC group. In this study, 78 individuals with postnatal closure (mean age, 7.4 years) and 76 individuals with fMMC (mean age, 7.5 years) were included [13].
Clayton et al discovered that management of neurogenic bowel and bladder in individuals after fMMC remains a major problem. At a mean age of 9.6 years, 82.1% of individuals needed clean intermittent catheterization to control their bladders, 85.7% needed bowel management to deal with constipation, and 21.4% had lower urinary tract reconstruction surgery that included enterocystoplasty and a catheterizable bladder channel. When videourodynamics were conducted on half of the individuals, at a mean age of 7.4 years, it was discovered that 71% of the individuals had lower bladder capacity, 35% had overactive detrusors, and 25% had elevated detrusor pressure. No discernible differences in bladder management, urinary tract surgery, or urodynamics were seen when compared to individuals of the same age and sex who had postnatal closure [14].
fMMC individuals results in a higher percentage of individuals who have achieved continence than the postnatal group, according to the study of Carr [15] in pre-Management of Myelomeningocele Study (MOMS) trial individuals with follow-up for 5 years following fMMC, 18.5% of individuals have efficaciously trained their toilets, whereas 3.7% have bowel continence, 1.8% have bladder continence but need enemas, and 3.7% of individuals who successfully trained their toilets had spinal dermoid cysts that need to be surgically removed [15].
Incontinence between catheterizations, the requirement for clean intermittent catheterization, or the use of anticholinergic/ antibiotic medications did not differ across the groups, according to research by Lee et al. [16] about whether fMMC enhances lower urinary tract function. Detrusor overactivity, bladder capacity, detrusor pressure at capacity, and the presence of detrusor sphincter dyssynergia were all urodynamic parameters that did not substantially differ across the groups. The rates of ventriculoperitoneal shunting and untethering operation were identical. fMMC group had a mean follow-up of 7.2 years, while the postnatal group had a mean follow-up of 7.3 years. For fMMC and postnatal closure, the average individual age at comparable urodynamic examinations was 5.9 years and 6.0 years, respectively. In the postnatal closure cohort, the fMMC group consisted of 5 lumbar and 6 sacral level abnormalities with equal matching [16].
With a mean age of 7.4 years, Brock et al. [17] investigated the effectiveness of fMMC on urological outcomes at school age. With 24% of the fMMC group reporting voluntary voiding compared to 4% of the postnatal group, they discovered that the voiding status was significantly different across the groups. The 2 groups did not differ in terms of vesicostomy, urethral dilatation, or augmentation cystoplasty. Except for a larger postvoid residual urodynamic catheterization volume, there were no other statistically significant changes between the renal/ bladder ultrasonography results and the videourodynamic data. Overall, 62% of prenatal surgery patients and 87% of postnatal surgery patients were put on clean intermittent catheterization, respectively [17].
fMMC is associated with a better long-term QoL in comparison to postnatal closure, according to Mummareddy et al. [18] Individuals who received fMMC had considerably better psychological health than the postnatal group. Between the 2 groups, there were not statistically substantial differences in physical health, number of procedures performed on the individuals, and proportion of individuals who underwent nonneurosurgical operations. Neurosurgical procedures were performed on fewer individuals in the fMMC group than in the postnatal group. There were also no differences between the intrauterine group and the postnatal group in terms of age (median: 17 years vs. 15 years), sex, or lesion level [18].
The most frequent type of spina bifida, known as myelomeningocele, is characterized by the spinal cord and meninges protruding through a defect in the vertebral column [19]. Myelomeningocele is an expensive lifelong condition with numerous comorbidities such as sensory and motor lower limb impairment, bladder/bowel dysfunction, scoliosis, club foot, and hydrocephalus [20].
The incidence of myelomeningocele in the United States has stabilized at 3.4 per 10,000 live births despite folic acid fortification. Liveborn infants with myelomeningocele have a 10% mortality rate [21]. Long-term survivors experience lifelong morbidities, such as hydrocephalus, Chiari II malformation, bladder and bowel problems, and some degree of paralysis of the lower extremities. Even with early postnatal surgical treatment, damage to the spinal cord and peripheral nerves is commonly present at birth and permanent. The degree of spinal cord injury affects how severely the lower limbs are neurologically impaired [21,22].
During regular antenatal examinations, fetal myelomeningocele can be diagnosed. Prenatal ultrasound is comparable to prenatal magnetic resonance imaging for the diagnosis of open spina bifida. At 12–14 weeks of gestation, severe spina bifida can now be identified early because of advancements in ultrasound technology [1,23]. When myelomeningocele is conclusively detected during pregnancy, the conventional course of treatment is to abort the pregnancy or get prepared for postnatal surgery. Postnatal surgery is performed to cover the exposed spinal cord and avoid from future spinal cord deterioration. If hydrocephalus is detected, a ventricular shunt is also inserted. The ideal window for surgical management is between 24–48 hours following delivery and should be performed within 72 hours of the patient's arrival to avoid the risk of infection and neurological damage. If postnatal surgery is not performed, the survival rate to 2 years of age is only 20%. Postnatal surgery can stop the myelomeningocele from progressing worse, but it cannot reverse the current damage of spinal cord. The process of neurologic damage, which begins with abnormal neurulation and continues throughout gestation, made myelomeningocele a better option for fetal closure surgery [2,6,24-28].
The surgical approaches for fMMC are open, fetoscopic, and a combination of both. The open approach entails a maternal laparotomy, exteriorization of the uterus, and a hysterotomy. The fetoscopic approach entails inserting fetoscopic ports directly into the uterus and abdominal wall of the mother. Like the open procedure, the combined approach entails uterine exteriorization following a maternal laparotomy, but fetoscopic ports are inserted through the uterus rather than a hysterotomy [29].
To compare the effectiveness of prenatal versus postnatal myelomeningocele closure, a randomized controlled trial known as the MOMS was carried out in 2011 at 3 maternal-fetal institutions in the United States. The prognosis was enhanced by performing fMMC prior to 26 weeks of pregnancy, which decreased the risk of mortality or requiring shunting by 12 months of age and reduced the degree of hindbrain herniation linked to Chiari II malformation. fMMC also enhanced the composite score for motor function and mental development at 30 months of age. Given the substantial advantage that fMMC had over postnatal closure, the experiment was ended early based on the effectiveness of fMMC in these first outcomes [21].
In terms of long-term neurocognitive and neurodevelopmental outcomes, this systematic review revealed that most fMMC individuals had average preschool neurodevelopmental scores, had attained cognitive and movement independence but still needed a lot of help with self-care. Individuals with fMMC who did not require shunt installation tended to perform better [8,9]. In term of long-term neurobehavioral outcome, preterm delivery and fMMC have not been linked to an increase in behavioral issues, impaired social interactions, or restricted behavior patterns [10,11]. In term of long-term neurofunctional outcome, fMMC surgery was revealed to enhance long-term functional outcomes that the fMMC group tended to be community ambulators and had better self-care skills and gait quality, performed the 10-m walk test 1 second faster, and were able to perform higher-level mobility skills [12,13]. And in term of the long-term QoL outcome, individuals who received fMMC had considerably better psychological health than the postnatal group [18]. However, research on the long-term outcomes of fMMC is still limited. Most research are limited by sample size and methodological differences; further large-scale research are required to determine the long-term outcomes following fMMC.
Preterm labor is one of the most common complications associated with fMMC. Prematurity of any age is a strong predictor of cognitive impairment that persists at various age [30]. Farmer et al. [31] found there was no difference in cognitive between fMMC group and postnatal group after 30-month follow-up, even though the prematurity rate in fMMC group was significantly higher than in postnatal group.
Reducing hydrocephalus rate is one of the most consistent findings in fMMC [32]. It might be the most beneficial aspects of fMMC since Rendeli et al. [33] and Iddon et al. [34] reported that spina bifida patients would have notable worse cognitive function if hydrocephalus was also developed, even it was treated. Furthermore, in current report, hydrocephalus in myelomeningocele patients was documented occurred in up to 77% patients [35]. Ventriculoperitoneal shunting is the main modality of treatment for hydrocephalus in myelomeningocele, but this procedure poses risk of shunt complications, such as blockage and infection [36]. It is suggested that neurodevelopmental impairment in shunted patients is related to shunt-related complication [37]. Endoscopic third ventriculostomy is another choice in treating hydrocephalus, but the success rate is very low if conducted in the first 48 hours after birth [38].
When compared to postnatal closure, fMMC was linked with greater risks of obstetrical complications such as oligohydramnios, chorioamniotic membrane separation, placental abruption, premature rupture of membranes, preterm delivery, and uterine scar dehiscence [19,39]. Johnson et al. [40] reported that there are increased risk of olygohydramnios, spontaneous membrane rupture, and spontaneous labors with relative risk of 6.40 (1.96–20.89), 5.78 (2.73–12.22), and 3.03 (1.74–5.29) respectively [40]. However, Farmer et al. [31] reported that there was no difference in mortality between postnatal and fMMC group in 30 months of age. Even so, concern must be made regarding the subsequent pregnancy. Though the risk of early fetal loss is similar to normal population, the risk of uterine dehiscence and rupture is significantly increased in subsequent pregnancy [41]. Adequate collaborative care should be addressed in planning subsequent pregnancy.
To date, there is no solid evidence regarding which patients are the best candidates for fMMC and which approach should be favored under certain conditions [42]. However, the advantages of fMMC have been described robustly in radiological or clinical parameters [32]. When trying to obtain parental approval for fMMC, clinicians must explain not only the advantages of fMMC for the fetus, but also the potential and relatively substantial risk for pregnancy complications that are not evident in postnatal repair. Future trials should focus on minimizing the rate of maternal and pregnancy complication after prenatal surgery.
In conclusion, most of the research revealed that fMMC provided substantial effects in enhancing long-term neurological cognitive, behavioral, functional, and QoL outcomes. However, most research are limited by sample size and methodological differences; further large-scale research are required to determine the long-term outcomes following fMMC.
Footnotes
Table 1.
PICO formulation
Table 2.
List of articles included in the systematic review
| Study | Year | Title | Study design | Subject | Authors’ conclusion | Risk of bias |
|---|---|---|---|---|---|---|
| Houtrow et al. [13] | 2021 | Prenatal repair and physical functioning among children with myelomeningocele: a secondary analysis of a randomized clinical trial | Randomized controlled trial | 154 | The advantages of FMMC confirmed at 30 months of age associated with mobility task (such as advance motor skill and sself-care) stayed further into school years. | Low |
| Houtrow et al. [11] | 2020 | Prenatal repair of myelomeningocele and school-age functional outcomes | Randomized controlled trial | 161 | Overall adaptive functioning did not differ significantly between surgical groups. FMMC's long-term advantages included improved mobility and independent performance, as well as fewer surgeries for ventriculoperitoneal shunting and revision. However, there was no convincing evidence that cognitive performance was enhanced. | Low |
| Brock et al. [17] | 2019 | Effect of prenatal repair of myelomeningocele on urological outcomes at school age | Randomized controlled trial | 156 | FMMC was associated with fewer reports of intermittent urinary catheterization at school years. In addition, caregiver reports indicate that children who experienced FMMC may be more probable to urinate on their own than those who experienced postnatal closure. | Low |
| Mummareddy et al. [18] | 2019 | Intrauterine closure of myelomeningocele is associated with superior long-term quality of life than postnatal closure: a single-center study | Cohort study | 23 | Children who experienced FMMC demonstrated superior psychosocial and overall quality of life metrics over the long-term compared to postnatal group. Due to the small sample size, not all medical and social economic confounding factors were accounted for; therefore, these findings require validation in a larger group across several centers. | Serious |
| Danzer et al. [12] | 2016 | Long-term neurofunctional outcome, executive functioning, and behavioral adaptive skills following fetal myelomeningocele surgery | Cohort study | 42 | FMMC surgery helps to improve long-term functional results. A significant proportion of FMMC children are capable of completing daily tasks, both at home and school. BAS abnormalities seem to to be more prevalent than EF deficits. FMMC children without a ventriculoperitoneal shunting who experience normal early development of the brain would be less likely to have issues with EF and BAS. FMMC surgery enhances long-term ambulation. Functional impairment is associated with symptomatic spinal cord tethering with or without an intradural cyst. More FMMC children are continent, still with a notable challenge on bladder and bowel control | Moderate |
| Carr [15] | 2015 | Urological results after fetal myelomeningocele repair in pre-MOMS trial patients at the Children's Hospital of Philadelphia | Cohort study | 54 | FMMC has resulted in a greater proportion of patients achieving continence than postnatal repair. | Moderate |
| Lee et al. [16] | 2012 | In utero closure of myelomeningocele does not improve lower urinary tract function | Cohort study | 33 | FMMC has been shown to reduce ventriculoperitoneal shunting and increase motor function, but it is not related to a significant progress in urinary tract function compared to postnatal repair group. | Moderate |
| Danzer et al. [9] | 2011 | Fetal myelomeningocele surgery: preschool functional status using the Functional Independence Measure for children (WeeFIM) | Cohort study | 26 | A significant proportion of children who underwent fMMC have attained mobility and cognitive independence, but still need sub-stantial assistance. Children without a ventriculoperiteoneal shunt history and those with normal neurodevelopmental results were more probable to be independent in daily life tasks. | Moderate |
| Danzer et al. [10] | 2011 | Preschool neurobehavioral outcome following fetal myelomeningocele surgery | Cohort study | 22 | fMMC surgery and premature birth are not related to an increase in behavioral issues, difficulty with social connections, or limited behaviors. FMMC children who also underwent shunt surgery were more probable to have results in 'at-risk' or 'clinically significant' spectrum for CBCL subindices. Early diagnosis of behavioral issues following fMMC surgery offers the chance for focused and timely intervention, allowing children who underwent FMMC to achieve social success. | Serious |
| Clayton et al. [14] | 2011 | Long-term urological impact of fetal myelomeningocele closure | Cohort study | 28 | After FMMC, neurogenic bowel and bladder management are still important concerns for patients. Closed monitoring should be performed in both fMMC and postnatal closure groups. | Moderate |
| Danz er et al. [8] | 2010 | Preschool neurodevelopmental outcome of children following fetal myelomeningocele closure | Cohort study | 30 | The majority of children who underwent fMMC had average developmental outcomes on preschool period. Children with history of fMMC who did not need ventriculoperitoneal shunt insertion were more probable to achieve higher scores. | Moderate |
References
1. Paslaru FG, Panaitescu AM, Iancu G, Veduta A, Gica N, Paslaru AC, et al. Myelomeningocele surgery over the 10 years following the MOMS trial: a systematic review of outcomes in prenatal versus postnatal surgical repair. Medicina 2021;57:707.



2. Sutton LN. Fetal surgery for neural tube defects. Best Pract Res Clin Obstet Gynaecol 2008;22:175–88.


3. Bauer DF, Beier AD, Nikas DC, Assassi N, Blount J, Durham SR, et al. Congress of neurological surgeons systematic review and evidence-based guideline on the management of patients with myelomeningocele: whether prenatal or postnatal closure affects future ambulatory status. Clin Neurosurg 2019;85:E409–11.

4. Kabagambe SK, Chen YJ, Vanover MA, Saadai P, Farmer DL. New directions in fetal surgery for myelomeningocele. Childs Nerv Syst 2017;33:1185–90.



5. Walsh DS, Adzick NS, Sutton LN, Johnson MP. The rationale for in utero repair of myelomeningocele. Fetal Diagn Ther 2001;16:312–22.



6. Moldenhauer JS, Adzick NS. Fetal surgery for myelomeningocele: after the Management of Myelomeningocele Study (MOMS). Semin Fetal Neonatal Med 2017;22:360–6.


7. Resnik DB. Beyond post-marketing research and MedWatch: long-term studies of drug risks. Drug Des Devel Ther 2007;1:1–5.




8. Danzer E, Gerdes M, Bebbington MW, Zarnow DM, Adzick NS, Johnson MP. Preschool neurodevelopmental outcome of children following fetal myelomeningocele closure. Am J Obstet Gynecol 2010;202:450. e1-450.e9.

9. Danzer E, Gerdes M, Bebbington MW, Koh J, Adzick SN, Johnson MP. Fetal myelomeningocele surgery: preschool functional status using the Functional Independence Measure for children (WeeFIM). Childs Nerv Syst 2011;27:1083–8.



10. Danzer E, Gerdes M, Bebbington MW, Koh J, Adzick NS, Johnson MP. Preschool neurobehavioral outcome following fetal myelomeningocele surgery. Fetal Diagn Ther 2011;30:174–9.



11. Houtrow AJ, Thom EA, Fletcher JM, Burrows PK, Scott Adzick N, Thomas NH, et al. Prenatal repair of myelomeningocele and school-age functional outcomes. Pediatrics 2020;145:e20191544.



12. Danzer E, Thomas NH, Thomas A, Friedman KB, Gerdes M, Koh J, et al. Long-term neurofunctional outcome, executive functioning, and behavioral adaptive skills following fetal myelomeningocele surgery. Am J Obstet Gynecol 2016;214:269. e1-269.e8.

13. Houtrow AJ, MacPherson C, Jackson-Coty J, Rivera M, Flynn L, Burrows PK, et al. Prenatal repair and physical functioning among children with myelomeningocele: a secondary analysis of a randomized clinical trial. JAMA Pediatr 2021;175:e205674.



14. Clayton DB, Tanaka ST, Trusler L, Thomas JC, Pope JC 4th, Adams MC, et al. Long-term urological impact of fetal myelomeningocele closure. J Urol 2011;186(4 Suppl): 1581–5.


15. Carr MC. Urological results after fetal myelomeningocele repair in preMOMS trial patients at the Children’s Hospital of Philadelphia. Fetal Diagn Ther 2015;37:211–8.



16. Lee NG, Gomez P, Uberoi V, Kokorowski PJ, Khoshbin S, Bauer SB, et al. In utero closure of myelomeningocele does not improve lower urinary tract function. J Urol 2012;188(4 Suppl): 1567–71.


17. Brock JW, Thomas JC, Baskin LS, Zderic SA, Thom EA, Burrows PK, et al. Effect of prenatal repair of myelomeningocele on urological outcomes at school age. J Urol 2019;202:812–8.


18. Mummareddy N, Dewan MC, Huang A, Basem J, Bennett KA, Shannon CN, et al. Intrauterine closure of myelomeningocele is associated with superior long-term quality of life than postnatal closure: a single-center study. J Neurosurg Pediatr 2019;24:115–9.


19. Kabagambe SK, Jensen GW, Chen YJ, Vanover MA, Farmer DL. Fetal surgery for myelomeningocele: a systematic review and meta-analysis of outcomes in fetoscopic versus open repair. Fetal Diagn Ther 2018;43:161–74.



20. Cavalheiro S, da Costa MDS, Moron AF, Leonard J. Comparison of prenatal and postnatal management of patients with myelomeningocele. Neurosurg Clin N Am 2017;28:439–48.


21. Adzick NS, Thom EA, Spong CY, Brock JW, Burrows PK, Johnson MP, et al. A randomized trial of prenatal versus postnatal repair of myelomeningocele. N Engl J Med 2011;364:993–1004.



22. Laskay NMB, Arynchyna AA, McClugage SG, Hopson B, Shannon C, Ditty B, et al. A comparison of the MOMS trial results to a contemporaneous, single-institution, postnatal closure cohort. Childs Nerv Syst 2017;33:639–46.



23. Munoz JL, Bishop E, Reider M, Radeva M, Singh K. Antenatal ultrasound compared to MRI evaluation of fetal myelomeningocele: a prenatal and postnatal evaluation. J Perinat Med 2019;47:771–4.


24. Northrup H, Volcik KA. Spina bifida and other neural tube defects. Curr Probl Pediatr 2000;30:313–32.


25. Adzick NS. Fetal myelomeningocele: natural history, pathophysiology, and in-utero intervention. Semin Fetal Neonatal Med 2010;15:9–14.


26. Bannister CM. The case for and against intrauterine surgery for myelomeningoceles. Eur J Obstet Gynecol Reprod Biol 2000;92:109–13.


27. Mitchell LE, Scott Adzick N, Melchionne J, Pasquariello PS, Sutton LN, Whitehead AS. Spina bifida. Lancet 2004;364:1885–95.


28. Caldarelli M, di Rocco C. Myelomeningocele primary repair surgical technique. In: Ozek MM, Cinalli G, Maixner W, editors. The spin bifida: management and outcome. Milan: Springer, 2008:143–55.
29. Yamashiro KJ, Farmer DL. Fetal myelomeningocele repair: a narrative review of the history, current controversies and future directions. Transl Pediatr 2021;10:1497–505.



30. Allotey J, Zamora J, Cheong-See F, Kalidindi M, Arroyo-Manzano D, Asztalos E, et al. Cognitive, motor, behavioural and academic performances of children born preterm: a meta-analysis and systematic review involving 64 061 children. BJOG 2018;125:16–25.



31. Farmer DL, Thom EA, Brock JW, Burrows PK, Johnson MP, Howell LJ, et al. The management of myelomeningocele study: full cohort 30-month pediatric outcomes. Am J Obstet Gynecol 2018;218:256. e1-256.e13.

32. Sacco A, Ushakov F, Thompson D, Peebles D, Pandya P, De Coppi P, et al. Fetal surgery for open spina bifida. Obstet Gynaecol 2019;21:271–82.




33. Rendeli C, Ausili E, Moroni R, Capriati M, Massimi L, Zanetti C. Neuropsychological profiles in children and young adults with spina bifida. Childs Nerv Syst 2021;37:2033–8.




34. Iddon JL, Morgan DJR, Loveday C, Sahakian BJ, Pickard JD. Neuropsychological profile of young adults with spina bifida with or without hydrocephalus. J Neurol Neurosurg Psychiatry 2004;75:1112–8.



35. McCarthy DJ, Sheinberg DL, Luther E, McCrea HJ. Myelomeningocele-associated hydrocephalus: nationwide analysis and systematic review. Neurosurg Focus 2019;47:E5.


36. Norkett W, McLone DG, Bowman R. Current management strategies of hydrocephalus in the child with open spina bifida. Top Spinal Cord Inj Rehabil 2016;22:241–6.



37. Danzer E, Finkel R, Gerdes M, Schwartz EMS, Rintoul NN, Adzick NS, et al. The relationship of seizure activity and chronic epilepsy in early infancy and short-term neurodevelopmental outcome following fetal myelomeningocele closure. Neuropediatrics 2010;41:140–3.


38. el Damaty A, Marx S, Cohrs G, Vollmer M, Eltanahy A, el Refaee E, et al. ETV in infancy and childhood below 2 years of age for treatment of hydrocephalus. Childs Nerv Syst 2020;36:2725–31.




39. Kabagambe SK, Chen YJ, Farmer DL. Fetal surgery for myelomeningocele: current clinical practice and translational research. Minerva Pediatr 2016;69:59–65.


40. Johnson MP, Bennett KA, Rand L, Burrows PK, Thom EA, Howell LJ, et al. The Management of Myelomeningocele Study: obstetrical outcomes and risk factors for obstetrical complications following prenatal surgery. Am J Obstet Gynecol 2016;215:778. e1-778.e9.








 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link PubMed
PubMed Download Citation
Download Citation


