Article Contents
| Clin Exp Pediatr > Volume 66(11); 2023 |
|
Abstract
An estimated 15 million infants are born prematurely each year. Although the survival rate of preterm infants has increased with advances in perinatal and neonatal care, many still experience various complications. Since improving the neurodevelopmental outcomes of preterm births is a crucial topic, accurate evaluations should be performed to detect infants at high risk of cerebral palsy. General movements are spontaneous movements involving the whole body as the expression of neural activity and can be an excellent biomarker of neural dysfunction caused by brain impairment in preterm infants. The predictive value of general movements with respect to cerebral palsy increases with continuous observation. Automated approaches to examining general movements based on machine learning can help overcome the limited utilization of assessment tools owing to their qualitative or semiquantitative nature and high dependence on assessor skills and experience. This review covers each of these topics by summarizing normal and abnormal general movements as well as recent advances in automatic approaches based on infantile spontaneous movements.
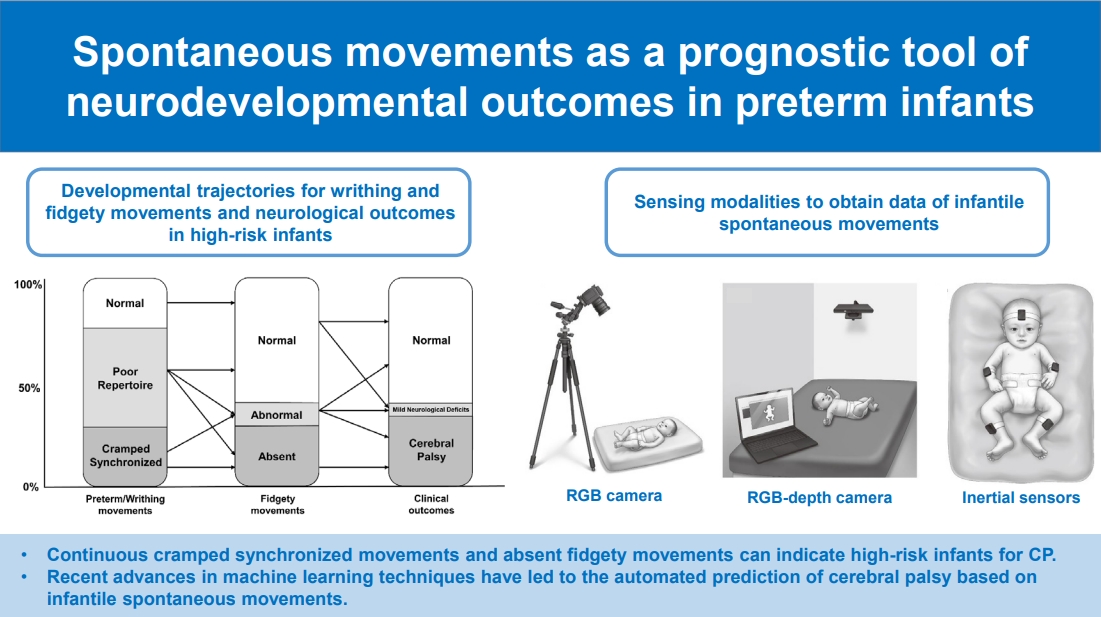
Graphical abstract. CP, cerebral palsy; RGB, red-green-blue.
The global incidence of preterm infants is approximately 15 million per year [1]. Preterm birth is a leading cause of neonatal death and closely associated with multiple serious complications such as bronchopulmonary dysplasia, chronic lung disease, retinopathy of prematurity, and intraventricular hemorrhage [2]. Notably, the impact of complications in preterm infants can extend into childhood or adulthood. One such major medical condition, cerebral palsy (CP), is the most common motor disability and presents with permanent abnormal movements, muscle tone, and posture. The overall prevalence of CP ranges from 1.5 to more than 4 per 1,000 live births worldwide [3]. Early intervention should be considered for high-risk infants with CP. The rate of rehabilitation support before 1 year of age in Korea is 34.9%–37.6% [4].
Accurate evaluations should be performed to detect infants at high risk of CP and provide rehabilitation support. Early detection in preterm infants before 6 months of corrected age is emphasized, and several examination tools have been recommended to enable early screening including the Hammersmith Infant Neurological Examination, brain magnetic resonance imaging (MRI), and the General Movements Assessment (GMA) [5]. In particular, the GMA is highly useful for identifying motor dysfunction based on infant’s quality of spontaneous movements (95%–98% predictive of CP) combined with neuroimaging [5]. The GMA is a validated standardized motor assessment used to evaluate video recordings of spontaneous movements of infants <5 months of corrected age [6]. Spontaneous movements can be analyzed to identify abnormal writhing movements or absent or abnormal fidgety movements.
This narrative review aimed to summarize spontaneous movements in healthy infants and their relation to brain development and neural substrates; discuss early markers of spontaneous movements to detect high-risk infants based on the GMA; review recent advances in automatic approaches used to examine infantile spontaneous movements; and suggest the current limitations of assessments based on infantile spontaneous movements and directions for future research.
Spontaneous movements are considered a window for the early identification of high-risk infants from the fetal to postterm periods because they can reflect neurological integrity [7]. Unlike reflexes, spontaneous movements are not evoked by external stimuli. Prechtl [8] proposed an approach to the neurological evaluation of newborns by observing spontaneous movements based on Gestalt perception and named it general movements (GMs). Previous studies of fetuses and preterm and term infants demonstrated that GM assessment results are correlated with both brain lesions in terms of neuroimaging techniques such as cranial ultrasound or MRI and neurological outcomes [9,10]. More recently, Peyton et al. [11] showed that the presence of aberrant GMs at 10–15 weeks of corrected age is associated with adverse neurodevelopmental outcomes and specific white matter microstructural abnormalities contributing to cognitive, language, and motor delays.
Reflexes and responses evoked by external stimuli or physical examinations are relatively less sensitive for detecting brain lesions. Sherrington [12] isolated the spinal cord from the dominant brain and analyzed the mechanism of simple reflex behavior. In brainless animals, simple reflexes are provoked as automatic responses. In decerebrated animals, the quantitative relationship between sensory input and reflexive motor output can be analyzed. It is assumed that reflexes are not optimal indicators of brain integrity or damage, whereas spontaneous movements, such as the expression of spontaneous neural activity, are excellent markers of neural dysfunction caused by brain impairment.
In mammals and humans, there is a transient structure in the developing brain called neural subplate [13]. The complex and variable characteristics of GMs are assumed induced by modulating cortical subplate activity [14]. When the subplate gradually dissolves by up to 3 months of corrected age, the cortical plate dominates [15]. This is the time when fidgety movements first appear. When cortical activity in the primary sensorimotor cortices shifts from the subplate to the cortical plate at 2–5 months of corrected age, fidgety GMs can reveal cortical development [16]. In turn, the absence of fidgety movements highly indicates neural injury.
In a human fetus, variable specific movement patterns appear at 9–12 weeks’ postmenstrual age and are known to disappear at 1 year of age. These endogenously generated movements continue after birth, even in preterm infants [17]. GMs are endogenously operated on spontaneous movements that may last from a few seconds to several minutes. GMs were first addressed by Prechtl [8] The movements involve a sequential wave of the arm, hand, leg, foot, neck, and trunk with variable speed and intensity and a gradual beginning and end. The elegant rotations of the limbs and complex movements are usually unpredictable and variable [8]. Before term birth, we refer to them as fetal or preterm GMs [18]. From 40 weeks to approximately 46–49 weeks of postmenstrual age, they are called writhing movements [19]. GMs have a similar appearance from early fetal life until the end of 2 months of corrected age, although age-related minor differences exist. Writhing movements are characterized by small to moderate amplitudes at a slow to moderate speed. At 46–49 weeks of postmenstrual age, GMs with a writhing characteristic gradually disappear and fidgety GMs start to appear [6]. Fidgety movements are oscillating movements of small amplitude and moderate speed with variable acceleration of the neck, trunk, and limbs in all directions. Fidgety movements are present until the end of the first 6 months of life, when antigravity movements begin to dominate (Fig. 1).
The quality of GMs can change when the brain is impaired. Previous studies demonstrated that, in newborn infants with brain lesions, spontaneous movements lose variability, fluency, and elegance as opposed to a mere reduction in motility occurring [6]. The observer’s visual Gestalt perception is important for detecting alterations in sequential movements. At each stage, there are several types of abnormal movements with different predictive values and prevalence (Table 1).
Poor repertoire GMs are monotonic sequences of repertoires. The movements of different body parts showed repetitive patterns with less variability in speed and intensity, similar to normal writhing movements. Although the emergence of a poor repertoire does not necessarily indicate a poor prognosis, serial observations are necessary since they can be followed by normal, abnormal, or absent fidgety movements (Fig. 2) [6]. Cramped synchronized GMs are abnormal and have high predictive value for the outcome. These abnormal GMs appear rigid and lack normal smooth, variable, and fluent characteristics, while all limb and trunk muscles contract and relax almost simultaneously [6]. If this abnormal pattern is observed consistently or dominantly, it is highly predictive of CP [6,20-22]. Chaotic GMs can be observed during the preterm and term periods and even after 40 weeks of postmenstrual age but are rather rare. Movements with large amplitudes and fast speeds involve all limbs and occur in a chaotic order without fluency or smoothness. They consistently appear abrupt [23,24]. Chaotic GMs can often become cramped, synchronized GMs within a few weeks [18].
The absence of fidgety movements is highly predictive of later neurological impairments, particularly for CP [25]. If fidgety movements are never observed between 9 and 20 weeks of corrected age, this is referred to as “absence of fidgety movements.” Abnormal fidgety movements are rare and have low predictive value. While these movements resemble normal fidgety movements, their amplitudes, speeds, and jerkiness are either moderately or significantly exaggerated.
It is widely accepted that the GMA is a highly recommended method of predicting neurological outcomes of young infants along with neonatal MRI and the Hammersmith Infant Neurological Examination [5]. The GMA was previously compared with other neurological examinations or neuroimaging tools [22,26]. Cioni et al. [27] reported that the GMA showed superior sensitivity and specificity to traditional neurological examinations of the Amiel-Tison and Touwen criteria for neurological outcomes until 2 years of age. In this study, the results of the GMA were also highly correlated with neurological outcomes in infants. Another previous study indicated that continuous observations of cramped synchronized GMs showed greater sensitivity and specificity for predicting CP than Dubowitz and Touwen neurological examinations [22]. Additionally, a recent systematic review reported that the sensitivity of neonatal MRI and GMA was 86% and 98%, respectively, and their combination with history taking about risk factors is strongly recommended in high-risk newborns for the early detection of CP [28,29].
The role of the GMA can be extended to predict both motor developmental outcomes and cognitive or behavioral outcomes. A previous study showed that abnormal GMs were correlated with the development of attention-deficit-hyperactivity disorder as well as aggressive behavior [30]. In a prospective study of 31 infants without CP, the presence of abnormal GMs was related to the presence of attention-deficit-hyperactivity disorder [31]. Moreover, infants with autism spectrum disorder more often showed poor repertoire GMs as well as abnormal or absent fidgety GMs than a control group [32]. Likewise, in a review by Einspieler et al. [33], GMA results were highly associated with the later diagnosis of autism spectrum disorder or Rett syndrome. The quality of writhing and fidgety GMs in 17 children who were diagnosed with typical or atypical Rett syndrome showed abnormal GMs during their first few months of life [33].
Since GMs start at an early fetal age, it is essential to administer the GMA to high-risk infants before discharge from neonatal intensive care unit and throughout their development. The prognostic value of the GMA increases with serial observations [21,34]. It is usually recommended to conduct the GMA using several recordings of GMs from the preterm period to 8 weeks of corrected age, and at least one recording during the fidgety periods (9–16 weeks) [35].
The assessment is based on the overall visual Gestalt perception, and standardized training with the observer’s detailed skills is extremely important. GM observation is conducted by individuals who have received certification through 4- to 5-day training courses provided by the GMs Trust (http://www.general-movementstrust.info). It has been reported that approximately 9,000 videos assessed by 800 observers yielded a correct diagnosis rate of 83% after the completion of the training course [36]. Interscorer agreement reliability has a kappa value range of 0.81–0.92 [36-38].
Recent rapid advances in the fields of computer vision and pattern recognition based on machine learning techniques have led to the publication of numerous articles on automated approaches to GMA. Machine learning is a cutting-edge, datadriven approach that encompasses the intrinsic properties of the constituents of data. It has a core strength of predicting the expert level or, at least, providing supportive information to health professionals [39]. Previous evidence of risk factors or characteristics of spontaneous movements for CP in preterm infants implies that machine learning has great potential for the early detection of high-risk infants with CP [40].
Data acquisition from infantile spontaneous movements is practical using diverse types of sensing modalities. There are 2 categories of sensing modalities for acquiring data on infantile spontaneous movements: indirect (video cameras, 3-dimensional motion capture, and Microsoft Kinect) and direct (inertial sensors, magnet tracking system) [40]. Fig. 3 illustrates several representative sensing modalities used to record infantile spontaneous movements in previous studies [40,41]. The determination of sensing modalities is among the key steps for developing prediction models using infantile spontaneous movements because it can affect the essential properties of movements in each body region, quality, dimension, measurement error, and data acquisition ease.
The model performance for classifying infants at high risk of CP based on machine learning in previous studies seems to be satisfactory with an overall accuracy exceeding 80%–90% [42]. The majority of the developed prediction models utilized 2-dimensional video images, and some studies adopted 3-dimensional video images or data from inertial sensors. Table 2 sum marizes previous studies that developed machine learningbased prediction models or explored features to discriminate infants at low versus high risk of CP or neurologic impairment using 2-dimensional images of infantile spontaneous movements [43-47]. Although machine learning-based prediction models using infantile spontaneous movements showed promising results in previous studies, their clinical utility has not yet been demonstrated in clinical practice [42]. Most previous studies evaluated the model performance using only internal validation without reporting external validation results [42]. Thus, it is strongly recommended that future studies evaluate the performance of prediction models through external validation to confirm their clinical utility in the medical field [48].
Moreover, the discovery of biomarkers based on infantile spontaneous movements using machine learning enables us to understand the underpinnings of neurodevelopment and provides opportunities for health professionals to obtain novel insights. The exploration of novel features in developing machine learning-based prediction models can enhance model usability and help clinicians interpret and recognize them more confidently. Shin et al. [47] indicated that early neurological development in preterm infants is closely associated with movement complexity in terms of sample entropy at the upper and lower limbs, which is considered an important marker for a high risk of CP. This finding is consistent with the results of a previous study in which a lack of complexity might result from injury or dysfunction of the subplate or cortical plate and/or its connective fibers [49].
According to the guidelines, GMA is among the recommended evaluation tools for the early detection and intervention of preterm infants [5]. However, it is not universally utilized worldwide because of limited access to clinical expertise in GMA [50]. GMA is either a qualitative or semiquantitative evaluation method that is highly dependent on the skills and experience of the assessor. The assessment must be performed by certified individuals who have completed high-quality training courses [42]. Although GMA training courses are held several times a year, their regional distribution remains limited. Considering the privacy issues of educational content containing images of preterm infants, extending training courses by providing online courses for remote learners might be difficult despite the growing global acceptance of online courses. Automated approaches for GMA or prognostication using infantile spontaneous movements based on machine learning can be a good alternative to broaden its applicability [42]. These approaches may be less dependent on the skills or experiences of assessors and provide objective evaluations with quantitative results to support clinicians during the decision-making process for early intervention in clinical practice.
Spontaneous movements in preterm, term, and postterm infants reflect neuronal integrity and can be utilized to identify infants at high risk of developing CP. GMs are spontaneous movements involving the entire body, including the upper and lower extremities, neck, and trunk, before the emergence of voluntary or antigravity movements. In the GMA, abnormal writhing movements, which are categorized as poor repertoire, cramped synchronized, chaotic movements, and absent or abnormal fidgety movements, are highly predictive of CP or other neurological impairments. In particular, continuous cramped synchronized movements and the absence of fidgety movements indicate greater sensitivity and specificity for CP. Recent advances in machine learning techniques have led to the automated prediction of CP or neurological impairments based on infantile spontaneous movements. This may help overcome the limited utilization of the GMA owing to its qualitative or semiquantitative nature and high dependency on the skills and experience of the assessors.
Acknowledgments
This study was supported by Basic Science Research Program through the National Foundation of Korea (NRF) funded by the Ministry of Education (2020R1C1C1 010486) and by Biomedical Research Institute, Chung-Ang University Hospital. This study was also supported by the Seoul National University Hospital (04-2022-2050).
Fig. 1.
Development of general movements. General movements can be observed in fetuses from 9 weeks of postmenstrual age and gradually change to fidgety movements. Fidgety movements can be seen as early as 6 weeks of postterm age but usually occur around 9 weeks and are then present until 15 weeks (at most 20 weeks) of postterm age. When fidgety movements disappear, general movements are replaced by intentional movements.

Fig. 2.
Illustrations of developmental trajectories for writhing and fidgety movements and neurological outcomes in high-risk infants [6]. It is notable that cramped synchronized movements and absent fidgety movements indicate a high risk of cerebral palsy. Those who showed poor repertoire movements during the writhing period had different outcomes according to the characteristics of the fidgety movements. Those who showed abnormal fidgety movements can have different outcomes depending on the presentation of previous general movements.

Fig. 3.
Illustrations of sensing modalities to obtain data of infantile spontaneous movements: (A) red-green-blue (RGB) camera, (B) RGB-depth camera such as Microsoft Kinect, and (C) inertial sensors. An RGB camera is a popular device for obtaining 2-dimensional images for General Movements Assessment; recently, depth information was added to acquire 3-dimensional images using an RGB-depth camera. Inertial sensors, which consisting of accelerometers and gyroscopes, are utilized to continuously track the movements at the head, trunk, and upper and lower limbs.

Table 1.
Normal and abnormal general movements by age
Table 2.
| Study | Aim | Participants | Machine learning algorithm | Data | Feature | Performance or analyzing results |
|---|---|---|---|---|---|---|
| Groos et al. [46] (2022) | Prediction of cerebral palsy | N=557 | An ensemble of Graph Convolutional Networks | Timing of measurements: 9–18 weeks of corrected age | 1. Position | Accuracy: 90.6% |
| High risk of perinatal injury | 2. Velocity | Sensitivity: 71.4% | ||||
| 3. Distance from the neighboring body keypoints | Specificity: 94.1% | |||||
| - Preterm and not preterm | 19 Body key points (x, y) | PPV: 68.2% | ||||
| NPV: 94.9% | ||||||
| Sakkos et al. [45] (2021) | Identification of fidgety movements | N=37 | 1. OpenPose for detection of body parts | Timing of measurments: (-) | Trajectories of 8 selected body points (elbow, hand, knee, and ankle at the both side) | Accuracy: 90.2% |
| 1. MINI-RGBD synthetic dataset: 12 synthetically generated videos | Sensitivity: 83.3% | |||||
| 2. 1D CNN for spatial modeling | 14 Body key points (x, y) | Specificity: 94.7% | ||||
| Precision: 83.3% | ||||||
| 2. RVI-25 dataset: 25 videos from 25 different infants | 3. LSTM for temporal modeling | F1: 83.3% | ||||
| Shin et al. [47] (2022) | Development of automatic standardized methods for quantitatively analyzing spontaneous movements | N=65 | AlphaPose | Timing of measurments: 4 months of corrected age | 1. Complexity | Sample entropy as a complexity index of joint angles and angular velocities |
| 1. Gestational age <32 weeks | 2. Interlimb synchronization | |||||
| 2. Birth weight<1500 g | 12 Body key points (x, y) | |||||
| Doroniewicz et al. [44] (2020) | Detection of writhing movements on video recordings of children | N=31 | 1. OpenPose for detection of body parts | Timing of measurments: the second and third day of life | 1. Factor of movement's area: range | Accuracy: SVM (80.23), RF (80.93), LDA (80.41) |
| Healthy newborns, second or third day of life | Sensitivity: SVM (71.36), RF (44.18), LDA (39.70) | |||||
| 2. SVM, RF, LDA | 25 body key points (x, y) | 2. Factor of movement's shape - nature | Specificity: SVM (83.15), RF (93.02), LDA (93.81) | |||
| 3. Center of movement's area - location | AUC: SVM (0.83), RF (0.82), LDA (0.84) | |||||
| Ihlen et al. [43] (2019) | Development of a machine learning model to predict cerebral palsy and ambulatory function in children with cerebral palsy | N=377 with high-risk infants | Partial least square regression | Timing of measurments: 9–15 weeks of corrected age | Movement frequencies, amplitude, and covariations | Sensitivity: 92.7 |
| 12 Weeks of corrected age | Specificity: 81.6 | |||||
| 6 body key points (x, y) | AUC: 0.87 |
PPV, positive predictive value; NPV, negative predictive value; MINI-RGBD, Moving Infants In RGB-D; RVI, remote visual inspection; CNN, convolutional neural network; LSTM, long short-term memory; LDA, linear discriminant analysis; SVM, support vector machine; RF, random forest; AUC, area under the curve.
References
2. Siffel C, Hirst AK, Sarda SP, Kuzniewicz MW, Li DK. The clinical burden of extremely preterm birth in a large medical records database in the United States: mortality and survival associated with selected complications. Early Hum Dev 2022;171:105613.


3. Stavsky M, Mor O, Mastrolia SA, Greenbaum S, Than NG, Erez O. Cerebral palsy—trends in epidemiology and recent development in prenatal mechanisms of disease, treatment, and prevention. Front Pediatr 2017;5:21.



4. Lee JH, Youn Y, Chang YS, Network KN. Short-and long-term outcomes of very low birth weight infants in Korea: Korean Neonatal Network update in 2019. Clin Exp Pediatr 2020;63:284-90.




5. Novak I, Morgan C, Adde L, Blackman J, Boyd RN, BrunstromHernandez J, et al. Early, accurate diagnosis and early intervention in cerebral palsy: advances in diagnosis and treatment. JAMA Pediatr 2017;171:897-907.



6. Einspieler C, Prechtl HF. Prechtl’s assessment of general movements: a diagnostic tool for the functional assessment of the young nervous system. Ment Retard Dev Disabil Res Rev 2005;11:61-7.


7. Hadders-Algra M. General movements: a window for early identification of children at high risk for developmental disorders. J Pediatr 2004;145(2 Suppl): S12-8.


8. Prechtl HF. Qualitative changes of spontaneous movements in fetus and preterm infant are a marker of neurological dysfunction. Early Hum Dev 1990;23:151-8.


9. Sival D, Visser G, Prechtl HF. The effect of intrauterine growth retardation on the quality of general movements in the human fetus. Early Hum Dev 1992;28:119-32.


10. Prechtl HF, Ferrari F, Cioni G. Predictive value of general movements in asphyxiated fullterm infants. Early Hum Dev 1993;35:91-120.


11. Peyton C, Yang E, Msall M, Adde L, Støen R, Fjørtoft T, et al. White matter injury and general movements in high-risk preterm infants. Am J Neuroradiol 2017;38:162-9.



12. Sherrington CS. The integrative action of the central nervous system. New Haven (CT): Yale University Press, 1961.
13. de Graaf-Peters VB, Hadders-Algra M. Ontogeny of the human central nervous system: what is happening when? Early Hum Dev 2006;82:257-66.


14. Hadders-lgra M. Neural substrate and clinical significance of general movements: an update. Dev Med Child Neurol 2018;60:39-46.



15. Kerschensteiner D. Spontaneous network activity and synaptic development. Neuroscientist 2014;20:272-90.




16. Leighton AH, Lohmann C. The wiring of developing sensory circuits— from patterned spontaneous activity to synaptic plasticity mechanisms. Front Neural Circuits 2016;10:71.



17. Prechtl HF. Prenatal and early postnatal development of human motor behaviour. In: Kalverboer AF, Gramsbergen A, editors. Handbook of brain and behaviour in human development. Great Britain (UK): Kluwer Academic, 2001:415-27.
18. Einspieler C. Prechtl’s method on the qualitative assessment of general movements in preterm, term and young infants. Cambridge (UK): MacKeith Press, 2008.
19. Hopkins B, Prechtl HF. A qualitative approach to the development of movements during early infancy. In: Prechtl HF, editor. Continuity of neural functions from prenatal to postnatal life. Clinics in developmental medicine vol. 94. Oxford (UK): Blackwell Scientific Publications, 1984:179-97.
20. Prechtl HF, Einspieler C, Cioni G, Bos AF, Ferrari F, Sontheimer D. An early marker for neurological deficits after perinatal brain lesions. Lancet 1997;349:1361-3.


21. Ferrari F, Cioni G, Prechtl HF. Qualitative changes of general movements in preterm infants with brain lesions. Early Hum Dev 1990;23:193-231.


22. Ferrari F, Cioni G, Einspieler C, Roversi MF, Bos AF, Paolicelli PB, et al. Cramped synchronized general movements in preterm infants as an early marker for cerebral palsy. Arch Pediatr Adolesc Med 2002;156:460-7.


23. Bos AF, van Asperen RM, de Leeuw DM, Prechtl HF. The influence of septicaemia on spontaneous motility in preterm infants. Early Hum Dev 1997;50:61-70.


24. Ferrari F, Prechtl HF, Cioni G, Roversi MF, Einspieler C, Gallo C, et al. Posture, spontaneous movements, and behavioural state organisation in infants affected by brain malformations. Early Hum Dev 1997;50:87113.

25. Einspieler C, Peharz R, Marschik PB. Fidgety movements–tiny in appearance, but huge in impact. J Pediatr (Rio J) 2016;92:64-70.

26. Guzzetta A, Mercuri E, Rapisardi G, Ferrari F, Roversi M, Cowan F, et al. General movements detect early signs of hemiplegia in term infants with neonatal cerebral infarction. Neuropediatrics 2003;34:61-6.


27. Cioni G, Prechtl HF, Ferrari F, Paolicelli PB, Einspieler C, Roversi MF. Which better predicts later outcome in fullterm infants: quality of general movements or neurological examination? Early Hum Dev 1997;50:71-85.


28. Bosanquet M, Copeland L, Ware R, Boyd R. A systematic review of tests to predict cerebral palsy in young children. Dev Med Child Neurol 2013;55:418-26.


29. Ashwal S, Russman B, Blasco P, Miller G, Sandler A, Shevell M, et al. Practice parameter: diagnostic assessment of the child with cerebral palsy: report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology 2004;62:851-63.


30. Hadders-Algra M, Groothuis AM. Quality of general movements in infancy is related to neurological dysfunction, ADHD, and aggressive behaviour. Dev Med Child Neurol 1999;41:381-91.


31. Hadders-Algra M, Bouwstra H, Groen SE. Quality of general movements and psychiatric morbidity at 9 to 12 years. Early Hum Dev 2009;85:1-6.


32. Phagava H, Muratori F, Einspieler C, Maestro S, Apicella F, Guzzetta A, et al. General movements in infants with autism spectrum disorders. Georgian Med News 2008;(156):100-5.
33. Einspieler C, Sigafoos J, Bartl-Pokorny KD, Landa R, Marschik PB, Bölte S. Highlighting the first 5 months of life: general movements in infants later diagnosed with autism spectrum disorder or Rett syndrome. Res Autism Spectr Disord 2014;8:286-91.



34. Romeo DMM, Guzzetta A, Scoto M, Cioni M, Patusi P, Mazzone D, et al. Early neurologic assessment in preterm-infants: integration of traditional neurologic examination and observation of general movements. Eur J Paediatr Neurol 2008;12:183-9.


35. Einspieler C, Prechtl HF, Ferrari F, Cioni G, Bos AF. The qualitative assessment of general movements in preterm, term and young infants—review of the methodology. Early Hum Dev 1997;50:47-60.


36. Valentin T, Uhl K, Einspieler C. The effectiveness of training in Prechtl’s method on the qualitative assessment of general movements. Early Hum Dev 2005;81:623-7.


37. Bos AF, van Loon AJ, Hadders-Algra M, Martijn A, Okken A, Prechtl HF. Spontaneous motility in preterm, small-for-gestational age infants. II. Qualitative aspects. Early Hum Dev 1997;50:131-47.


38. Hadders-Algra M, Mavinkurve-Groothuis AM, Groen SE, Stremmelaar EF, Martijn A, Butcher PR. Quality of general movements and the development of minor neurological dysfunction at toddler and school age. Clin Rehabil 2004;18:287-99.



40. Marcroft C, Khan A, Embleton ND, Trenell M, Plötz T. Movement recognition technology as a method of assessing spontaneous general movements in high risk infants. Front Neurol 2015;5:284.



41. Schroeder AS, Hesse N, Weinberger R, Tacke U, Gerstl L, Hilgendorff A, et al. General Movement Assessment from videos of computed 3D infant body models is equally effective compared to conventional RGB video rating. Early Hum Dev 2020;144:104967.


42. Silva N, Zhang D, Kulvicius T, Gail A, Barreiros C, Lindstaedt S, et al. The future of General Movement Assessment: The role of computer vision and machine learning–a scoping review. Res Dev Disabil 2021;110:103854.



43. Ihlen EA, Støen R, Boswell L, de Regnier RA, Fjørtoft T, Gaebler-Spira D, et al. Machine learning of infant spontaneous movements for the early prediction of cerebral palsy: a multi-site cohort study. J Clin Med 2019;9:5.



44. Doroniewicz I, Ledwoń DJ, Affanasowicz A, Kieszczyńska K, Latos D, Matyja M, et al. Writhing movement detection in newborns on the second and third day of life using pose-based feature machine learning classification. Sensors 2020;20:5986.



45. Sakkos D, Mccay KD, Marcroft C, Embleton ND, Chattopadhyay S, Ho ES. Identification of abnormal movements in infants: a deep neural network for body part-based prediction of cerebral palsy. IEEE Access 2021;9:94281-92.

46. Groos D, Adde L, Aubert S, Boswell L, De Regnier RA, Fjørtoft T, et al. Development and validation of a deep learning method to predict cerebral palsy from spontaneous movements in infants at high risk. JAMA Network Open 2022;5:e2221325.



47. Shin HI, Shin HI, Bang MS, Kim DK, Shin SH, Kim EK, et al. Deep learning-based quantitative analyses of spontaneous movements and their association with early neurological development in preterm infants. Sci Rep 2022;12:3138.




48. Collins GS, Reitsma JB, Altman DG, Moons KG. Transparent reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): the TRIPOD statement. Ann Intern Med 2015;162:55-63.







 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link PubMed
PubMed Download Citation
Download Citation


