Article Contents
| Clin Exp Pediatr > Volume 66(11); 2023 |
|
Abstract
Long coronavirus disease (COVID), also known as postacute sequelae of severe acute respiratory syndrome coronavirus 2 infection, has been defined as signs and symptoms which persist for 4 weeks or even lasting for 6 months after the initial infection. Although the prevalence of long COVID in children is currently unknown, epidemiological investigations have reported cases in pediatric populations. Clinical manifestations of long COVID in children include respiratory symptoms, such as cough and dyspnea, as well as neuropsychiatric and general conditions, including fatigue, headache, and muscle weakness. The pathophysiology of long COVID in children is still being investigated, but potential mechanisms include viral persistence, autoimmunity, and neuroinflammation. Risk factors for long COVID in children are not yet well understood, but studies have suggested that children with a history of severe acute COVID-19 infection or comorbidities may be at increased risk. Evaluation for respiratory symptoms of long COVID in children is essential, including spirometry and imaging studies to assess lung function and any potential damage. Furthermore, long COVID in children has been associated with a higher prevalence of mental health problems than in adults, emphasizing the importance of monitoring and addressing these aspects in pediatric patients. Although our understanding of long COVID in children and adolescents is still evolving, it is clear that the condition can have significant impacts on their health and well-being. The aim of this review is to synthesize the current knowledge on the prevalence, risk factors, and pathophysiology of long COVID in children and adolescents, and to discuss potential management strategies based on existing evidence.
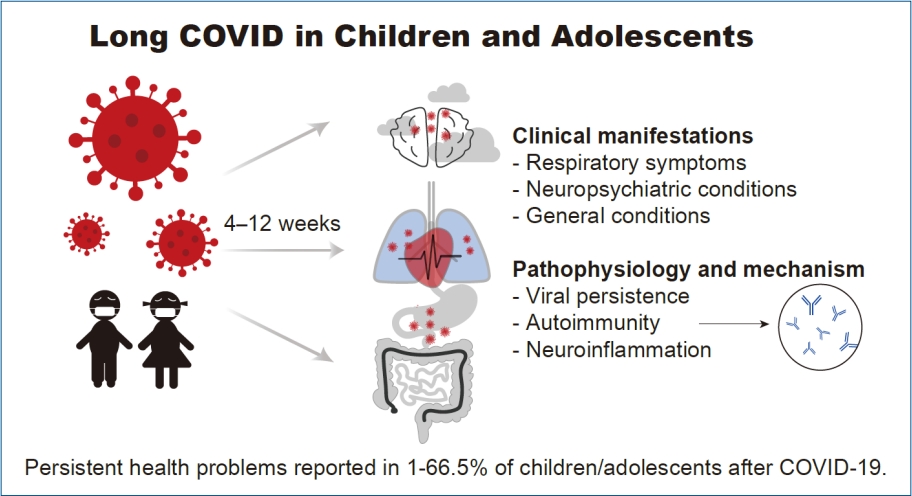
Graphical abstract. COVID-19, coronavirus disease 2019.
Long coronavirus disease (COVID) is a long-lasting condition persisting for weeks or even months, affecting individuals who had experienced or recovered from acute COVID 2019 (COVID-19) [1]. This condition, also known as postacute sequelae of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) or long-haul COVID, raised much concern as the ongoing COVID-19 transmission and new variants emerge [2]. Long COVID encompasses clinical manifestations beyond the period of acute infection that cannot be explained by an alternative diagnosis. While COVID-19 was generally asymptomatic or mild in children, greater susceptibility was presented to having long-lasting consequences in health. Considering that COVID-19 and related illness continues to exude major public health in children, knowledge of long COVID in this population is essential to guide in recognition and management [3]. Current data on long COVID prevalence in children is inconsistent, varying from 0% to 27.0% in different studies, and there is a lack of information in the pediatric population [4-9]. Moreover, there is a lack of consensus regarding the definition of long COVID, including the type, number, and duration of symptoms. Furthermore, while previous literature has discussed the mental and neuropsychiatric aspects of long COVID in children [10,11], there is a paucity of studies focusing on the respiratory aspects.
Therefore, our study aims to provide a comprehensive overview of long COVID in the pediatric population, including the prevalence, clinical manifestations, and management strategies, to enhance our understanding of this condition. Additionally, we will focus specifically on the epidemiology and management of respiratory aspects of long COVID in children to better address the health needs of this population.
The severity of acute SARS-CoV-2 infection in children is lower compared to the older population but children suffering from persistent symptoms after SARS-CoV-2 infection are raising concern. The Centers for Disease Control and Prevention defined of long COVID as illness beyond 4 weeks after initial infection [12]. The World Health Organization defined long COVID as continuation or new onset of symptoms 2 months after the initial SARS-CoV-2 infection, with no other explanation [13]. The National Institute for Health and Care Excellence recently identified long COVID including postacute and post COVID-19, with symptoms persisting for 4 to 12 weeks or for more than 12 weeks after onset of acute symptoms [14]. However, a definition for children or adolescents has not yet been established (Table 1).
Several studies have investigated symptoms of long COVID in children with persistent symptoms present 8–12 weeks after COVID-19 diagnosis [5,15,16]. The symptoms might or might not be related to the acute symptoms, but not present before infection. At least one persisting physical symptom that cannot be explained by an alternative diagnosis affects patients with this condition. The symptoms may interfere with daily functioning, or wax and wane over time.
The definition of long COVID in children is much less precisely defined than in adults. Meanwhile, the true prevalence of long COVID in children and adolescents is not well determined, with studies ongoing. According to previous literature and guidelines, evaluating the presence of symptoms between 4 and 12weeks after the acute COVID-19 infection is suggested appropriate. Children and adults are differently affected by the COVID-19 pandemic. Short-term complications were less severe in children, while long-term ones were worse such as impairing mental and physical activity, and daily well-being [17]. Thus, the impact of long-time imbalances related to COVID-19 affecting the pediatric population should not be underestimated [18]. The estimated incidence of children reporting COVID-related symptoms varies widely, mostly including symptoms such as fatigue, dyspnea, concentration difficulties, and headaches [18,19].
The earliest description of Long COVID was 5 children with Long COVID in Sweden. All 5 experienced mild symptoms during COVID-19 but suffered from persistent symptoms for 6–8 months after the infection [20,21]. A longitudinal cohort study in Swiss consisting of children with serology results conducted from October or November 2020 (Ciao Corona) reported that 4% of the seropositive children and 2% of the seronegative children reported any symptoms lasting beyond 12 weeks [22]. Another study in Italy examining children at 60 days from COVID-19 diagnosis from March to November 2020, evidenced symptoms present in 42.6% of the cases, and the most common symptom was insomnia followed by respiratory symptoms [9]. In January 2021, the Office for National Statistics (ONS) of England estimated that 12%–15% of children had symptoms at 5weeks, and 11.7% at 12 weeks after COVID-19 diagnosis, suggesting long COVID [23]. Recent studies that examined children at 4weeks from COVID-19 diagnosis estimated symptoms present in 4%– 9% of the cases [4,8,24,25], showing lower prevalence. The ONS soon updated the prevalence of long COVID in April 2021, estimating approximately 7%–8% of COVID-19 pediatric cases. Meanwhile, the estimated prevalence of long COVID have been widely variable among studies, reporting significantly higher prevalence rates of long COVID. A report from a retrospective cohort study during the month of July 2020 in Latvia identified that 30% reported persistent symptoms after COVID-19 recovery [26]. A prospective cohort study of children hospitalized with COVID-19 diagnosis between 2 April 2020 and 26 August 2020 diagnosed long COVID in 24.3% of the children assessed at 12weeks and common symptoms present included fatigue (10.7%) and sleep disturbances (6%) [27]. These discrepancies of these results may be attributed to analyses performed using pediatric long COVID definitions that are not yet drawn [21].
While the real-world prevalence remains uncertain and studies were also different in design, populations, follow-up durations, and methodology, emerging data suggest that there was an increase in health burden in children related to long COVID. The main findings of recently published epidemiological studies that examined the health burdens experienced by children after COVID-19 are presented in Table 2. The CLoCk Study [28], a large national study consisting of SARS-CoV-2 positive children between September 2020 and March 2021 in England, estimated that 66.5% of SARS-CoV-2 test positives and 53.3% of test negatives had at least one symptom at 3 months after SARS-CoV-2 testing suggesting long COVID. Among these children, 30.3% and 16.2% had 3 or more symptoms, respectively. In Denmark, epidemiological analyses (conducted on COVID-19 diagnosed children between January 2020 and July 2021 (LongCOVIDKidsDK), estimated that 61.9% of SARS-CoV-2 test positives and 57.0% of test negatives had at least one symptom at 2 months after SARS-CoV-2 testing. Participants in the case group reported better quality of life scores compared to the control group; however, they also reported a higher number of sick days and school absences. These studies reported that participants with SARS-CoV-2 test positives had more long-term symptoms with more burden of illness, while participants in the control group had more short-term symptoms and worse quality of life [15,29]. According to the estimates calculated by this cohort, 36.4%, 34.0%, 42.1%, and 45.2% of SARS-CoV-2 test positives aged 0–3, 4–11, 12–14, and 15–18 years had symptoms 829–1215 weeks after SARS-CoV-2 infection, respectively. Based on a large retrospective study which includes a matched control group, found that more than half of the children experienced long-term mental health consequences after COVID-19. These consequences were significantly higher than those seen in the control group, and persisted even after 12 months [30]. Another nationwide study that utilized register data from Norway and investigated 1.3 million children and adolescents, there was an increase in primary care utilization following COVID-19 infection, particularly for respiratory complaints and general or nonspecific conditions. This increase was most common within 4 weeks of infection but persisted for up to 6 months in children aged 1–5 years [31].
Measures of long COVID were limited to self-reported questionnaires, not clinically confirmed or validated [9,22,25,27,32]. A meta-analysis conducted on studies up to July 2021 has investigated the prevalence, risk factors, types, and duration of long COVID symptoms in children [33]. However, there is a need for reliable studies that compare the consequences of COVID-19 to those of other viral infections [34] in order to draw specific conclusions. Furthermore, the virus variants at the time of infection might affect the patterns of long-lasting symptoms, and also should be considered.
The clinical symptoms of long COVID in children varies, with one or more system involved (Table 3). As mentioned above, symptoms first reported 4–12 weeks after the acute infection are considered to be related to long COVID. The most common complaints of these children were fatigue, dyspnea, heart palpitations, and chest pain. The duration when children were assessed after a SARS-CoV-2 infection varies by studies, ranging from 4weeks to 6 months [20,24,33,35-37].
The most common complaints of the children first reported as long COVID were fatigue, dyspnea, heart palpitations, and chest pain, and subsequent studies have identified other symptoms such as cough, headache, and myalgia, as well as neurocognitive problems such as memory and concentration difficulties that can affect their daily life. Prevalence and severity of long COVID symptoms in children might differ within children by acute COVID-19 symptoms and age. Long COVID symptoms are more common in children with symptomatic or severe COVID-19; however, they are also described in asymptomatic patients [37].
Long-term respiratory symptoms after severe viral infection are limited to COVID-19, and postviral syndrome has been noticed. Despite that respiratory symptoms were not as frequent as in long COVID in adults, it is clear that they are frequently reported in children as well Previous studies have indicated that dyspnea and cough are common symptoms in children who test positive for SARS-CoV-2 [19,38]. In a recent prospective Cohort study conducted in Australia with a 3- to 8-week follow-up period, half of the symptomatic children experienced cough after being infected with SARS-CoV-2 [8]. Additionally, the most commonly reported persistent symptom in children with postacute sequelae of SARS-CoV-2 infection, according to a nationwide study in England (the CLoCk study), was sore throat [35]. Overall, dyspnea (13.8%–50%), cough (0.5%–5%), nasal symptoms (12.4%), sore throat (5.7%–80%), and chest pain (1.4%–31.1%) were identified as the commonly reported respiratory symptoms that lasted for an extended period after COVID-19.
Fatigue is one of the most common problems as in adults, and one study reported that up to 87% children with suspected long COVID suffered from this symptom [19]. Studies conducted against children demonstrated that common complaints in school-age children with long COVID were fatigue and headaches, while younger children were more affected by respiratory problems [39,40]. Compared to adults, children were less likely to complain long-term complaint after COVID-19 diagnosis.
Early reports of long COVID including a case series in children, reported that COVID-19 suspected patients developed long-term symptoms after acute infection, including fatigue, muscle pain, headache, concentration difficulties, palpitation, cough, and shortness of breath [9,20]. In 2020, a cohort study in Latvia that investigated symptoms of 9 children with long COVID reported that prolonged low-grade fever (22.22%), swollen lymph glands (11.11%), joint pain (11.11%), headache (11.11%), anosmia (11.11%), ageusia (11.11%), and microhaematuria (11.11%) was observed when assessed 101 (standard deviation, 17) days after the SARS-CoV-2 infection [26]. A Swedish study that assessed the persistent symptoms in 12 children 3 months after hospitalization due to COVD-19, fatigue (66.67%) was the most common symptom, followed by gastrointestinal (25%) and respiratory symptoms (25%); which all resolved after 6 months [41]. However, these studies of long COVID symptoms depended on self- or parent-reported symptoms, limited with restrictive measures, and absence of controls. Recently published studies that compared COVID-19 confirmed cases with controls showed higher rates of long-lasting symptoms, suggesting long COVID.
In an England study that matched 1,734 long COVID children with a same number of controls for age, sex, week of testing, fatigue (84.4%), headache (77.9%), and anosmia (77.9%) were shown 28 days after COVID-19 illness. In this study, school-age children and adolescents were more affected by mental and neurological disorders than preschoolers and younger children [25].
Similarly, fatigue was the most common symptom in a Latvian cross-sectional study that investigated persisting long COVID symptoms after 73.5 days (interquartile range, 41–110 days), with reported complaints 25.2%. Other neurocognitive sequelae were markedly frequent, such as irritability (24.3%), mood changes (23.3%), and headaches (16.9%) [39].
Fatigue (17.9%), concentration troubles (11.3%), and headache (9.1) and were also the most frequent symptoms in the case group of the LongCOVIDKidsDK (the Long COVID Kids Rapid Survey) study; a Danish survey investigating long COVID in children [15,42]. Results in line were reported in a controlled study investigating mental and physical health among English adolescents with long COVID (The CLoCk study). In this study, headache (39%) and tiredness (23%) were significantly reported in longCOVID cases after 2 months after testing positive for SARS-CoV-2, while differences in mental health and well-being scores were insignificant.
Another English study that used a national data, 9 symptoms were higher among the children after recovery from symptomatic SARS-CoV-2 infection. The significantly prevalent symptoms were: difficulty sleeping (8.8%), mood swings (7.8%), anxiety (7.8%), sadness (6.9%), confusion (5.6%), depression (4.1%), ageusia (5.3%), and anosmia (6.9%), and eye pain (2.8%). Notably, mental health problems were prevalent among all children in this study, despite negative test results, which implies the effect of the COVID-19 pandemic on the mental health of children [43].
Additional research is needed to determine whether the aforementioned long COVID symptoms observed in children are attributed to the viral infection itself or to the absence of social support resulting from school closures and lockdowns.
Studies provided clinical evidence that long COVID in children is pathological; however, the mechanism of this condition remains unclear. Long COVID's pathophysiology can be attributed to several factors, such as acute organ damage during the infection phase, lengthy organ recovery time, persistence of chronic inflammation, the nonspecific effects of hospitalization, sequelae of critical illness, postintensive care syndrome, complications arising from comorbidities, or adverse medication effects. The factor of chronic inflammation, which can lead to persistent symptoms in long COVID, can be explained as follows.
Persistent reservoirs of the virus in tissue or the persistence of viral genetic material as previously documented is one of the potential mechanisms underlying long COVID [44]. Possibility of viral persistence associated with long COVID symptoms even after severalmonths of infection has been reported in longitudinal studies [45,46]. SARS-CoV-2 RNA can persist in nasopharyngeal swabs for from 14 to 63 days after initial diagnosis [47]. There have been instances of extended gastrointestinal viral shedding in COVID-19 patients detected in the feces for up to 3 months [48]. Studies have documented that prolonged SARS-CoV-2 shedding in children is longer in the gastrointestinal tract than in the respiratory tract [49]. Furthermore, it is supposed that SARS-CoV2 could be latent in the central nervous system (CNS) [50] and immune system tissue or cell [50]. These studies estimated that SARS-CoV-2 persistence might last up to even months in the body is possible, which may induce immunopathy contributing to long COVID [51].
Immunologic response to SARS-CoV-2 has been suggested to play a key role, possibility resulting in organ damage and longlasting pathological sequelae. Previous studies have documented development of rare and uncommon autoantibodies after that SARS-CoV-2 infection. These autoantibodies directed against the angiotensin type 1 receptor possibly dysregulate the reninaldosterone-angiotensin system such as in severe bronchiolitis [52]. Other autoantibodies such as the type 1 interferons are possibility linked with the prolonged symptoms after COVID-19 [53]. Proinflammatory activity and inflammatory processes by the SARS-COV-2 protein damaging the lung, brain, and heart tissue resulting in long COVID sequelae has been addressed [54].
Recent evidence suggests that transient receptor potential (TRP) channels may play a role in the neuroinflammation associated with long COVID [55]. These channels are responsible for detecting various stimuli (including pain, temperature, inflammation, and taste) and controlling sensory processes like cough reflex, airflow, and mucus production [56]. In addition, they are found in different tissues throughout the body, including the central and peripheral nervous systems, the respiratory system, the gastrointestinal tract, the cardiovascular system, and the skin [57]. SARs-CoV-2 virus can upregulate TRP channels, either by direct activation or through immune responses, thus promote the release of proinflammatory cytokines and chemokines, leading to an inflammatory response [55,58]. Spices which were their agonists and desensitize TRPV1 or TRPA1 in afferent neurons, can improve respiratory symptoms in patients within 1 to 2 minutes. Furthermore, gabapentin neuromodulators are effective in patients with chronic cough by blocking subtypes of TRP channels [55].
Bradykinin, a proinflammatory peptide, is thought to contribute to neuroinflammation in long COVID by increasing vascular permeability and stimulating the release of proinflammatory cytokines and chemokines [59]. The mucosal ACE2 receptor plays a crucial role in regulating the metabolism of bradykinin. When SARS-CoV-2 viruses bind to ACE2 receptors, it can result in a decrease in ACE2 expression and an increase in bradykinin levels [60]. The inflammation response can activate bronchopulmonary C fibers in the vagus nerve, leading to peripheral cough hypersensitivity [61]. In addition, bradykinin may also induce gastrointestinal symptoms such as nausea, vomiting, diarrhea, and abdominal pain by affecting the gut mucosa in a similar proinflammatory way [59]. The disruption of the blood-brain barrier due to inflammation can also lead to neuronal damage and dysfunction in the CNS.
Last, it has been suggested that SARs-CoV-2 can affect the brainstem, either through direct penetration into the CNS or through an inflammatory/immune response [62]. SARs-CoV-2 is a neurotropic virus, and the brainstem has a relatively high expression of ACE2 receptors and neuropilin-1, which the virus can use to infect cells. Autopsy studies have found evidence of SARs-CoV-2 in the olfactory nerves and brainstem's medulla in deceased COVID-19 patients [63]. In addition, SARs-CoV-2 can induce neuroinflammation by activating microglia and astrocytes, as well as leukocyte infiltration. Other autopsy studies have also identified pronounced neurodegeneration in the brainstem, possibly due to a pathological immune response or SARs-CoV-2 invasion of the brainstem [64]. As the brain stem plays a crucial role in regulating various body function, including respiratory, cardiovascular, gastrointestinal, and neurological system, persistent brain stem dysfunction has associations with a range of symptoms and conditions in long COVID [65].
It is important to note that the mechanism is still being discussed and more research is needed to have a better understanding of the underlying causes of long COVID in children.
Risk factors for long COVID in children may comprise age, sex, multiple and severe symptoms during acute illness, as well as preexisting psychiatric and allergic disorders (Table 4). Nonetheless, further research is needed to establish such risk factors in pediatric population. Some studies suggest that older age is a risk factor for long COVID symptoms in children, while other studies reported conflicting results. For example, a populationbased study in Norway found that younger children (1–5 years) experienced longer-lasting symptoms than older children (6–19 years) [31]. One study documented that children above 6 years of age were at a higher risk of experiencing symptoms for more than 5 months [27], while another study reported that children over 12 years of age were more likely to have persistent symptoms for at least 28 days [25]. Nevertheless, several other studies have also reported that older groups of children are more likely to have severe or prolonged symptoms, indicating that age is a meaningful factor [18,24,35,38,39].
Multiple studies have identified female sex as a risk factor for long COVID in both children, as in adults [24,35]. Interestingly, in the first published case series of 5 children with long COVID, 4 were female [20]. Females were more likely to complain long-lasting symptoms at follow-up, irrespective of their COVID-19 status [15,39]. However, in some studies, no difference in risk was observed between males and females [8,38]. Hence, additional studies are needed to clarify the risks by sex in children.
Long COVID symptoms were also shown to be more frequent among patients with and severe disease and allergic comorbidity at the time of acute infection [4,27]. Underlying physical and mental problems has also been described as a possible risk factor [35]. Some studies noted that the severity and duration during acute infection were related with long COVID symptoms [9,38], although evidence is insufficient.
Long COVID poses burden on children and public health. Thus, caregivers and children should be educated about the condition beyond the acute phase, even if they were asymptomatic at the time of COVID-19 diagnosis. To date, there are no standardized questionnaires for the pediatric population to diagnose and monitor long COVID in children unlike in adults and elderly [66]. Detailed history and physical examination help with the diagnosis in children after SARS-CoV-2 infection. Symptom based investigations may be sufficient for most children with long COVID, and only a minority require further evaluation. Management of children with long COVID requires a comprehensive approach that takes into account their clinical history and examination, with symptom control and psychological support [14].
Long COVID in children may be broadly placed into different categories upon the predominant persistent manifested symptom: (1) respiratory symptoms (e.g., shortness of breath, sore throat, cough and anosmia), (2) neuropsychiatric conditions including mental, neurological problems (e.g., fatigue, headache, and brain fog), and (3) general extrarespiratory symptoms. Categorization of symptoms according to the involved systems helps to identifying the etiology. Peculiarly, new onset symptoms in children after recovery from COVID-19 should be properly addressed to rule out life threatening complications such as Multisystem Inflammatory Syndrome in Children, as highlighted in recent studies (Fig. 1) [67]. Considering the possible clinical scenario, therapeutic strategies using biomarkers of inflammation and tissue injury might be necessary to distinguishing this condition [68].
In addition, we should be aware that lockdown, school closures, limitations of seeing friends and even families due to the pandemic brings emotional distress to children and adolescents. Predisposed children or children with mental health problem might suffer from social isolation during the pandemic. We should not mistake children suffering from mental health problem during the pandemic for long COVID.
There is no standardized set of tests for persistent respiratory symptoms in children with long COVID suitable for everyone. However, in order to rule out other conditions or to avoid missing the patient's serious condition, holistic and person-centered workups must be performed (Fig. 1) [69].
In a primary care, physical examinations in accordance with the clinical history should be conducted to assess their breathing, as well as check for any other signs of respiratory distress. Furthermore, despite blood tests which include a full blood count, kidney, liver and thyroid function tests, inflammation markers, N-terminal pro B-type natriuretic peptide (NT-BNP) or BNP, and glycosylated hemoglobin might be suggested when an alternative diagnosis is suspected, these should be guided by the person’s symptoms and used to supplement a detailed holistic assessment. A chest x-ray might be considered, but should be used for a holistic evaluation of further treatment as it is not a good indicator of improvement. A sit-to-stand test has been proposed to assess physical functions that are affected by muscle strength, endurance, and balance. However, clinical judgement would be needed because it is not suitable for whom with chest pain or severe fatigue.
In high-level hospitals, additional blood tests, such as d-dimer and troponin, and an exercise tolerance test were considered. In addition, lung function test [70], cardiac investigation and further chest imaging using a high-resolution computed tomography scan or computed tomography pulmonary angiography should be considered as appropriate. Ventilation perfusion (VQ) scintigraphy and VQ single-photon emission computed tomography help to screen for ongoing lung perfusion complications [71].
Long COVID is a substantial health issue that affects a large proportion of children. It may result from various mechanisms, such as immune responses, tissue damage due to persistent viral reservoirs, and prompted lymphoproliferative responses. Proper clinical evaluation is crucial to identify the etiology and tailoring treatment. The management of affected children should comprise symptom control and psychological support. While the prognosis of these cases is generally good, monitoring of the long-term outlook is necessary as this disease is still new. Therefore, relevant guidelines for medical and psychological support for affected children during the COVID-19 pandemic are necessary.
Fig. 1.
Approach to children with persistent symptoms after COVID-19. COVID-19, coronavirus disease 2019; MIS-C, multisystem inflammatory syndrome in children; MRI, magnetic resonance imaging; SPECT, single-photon emission computed tomography.
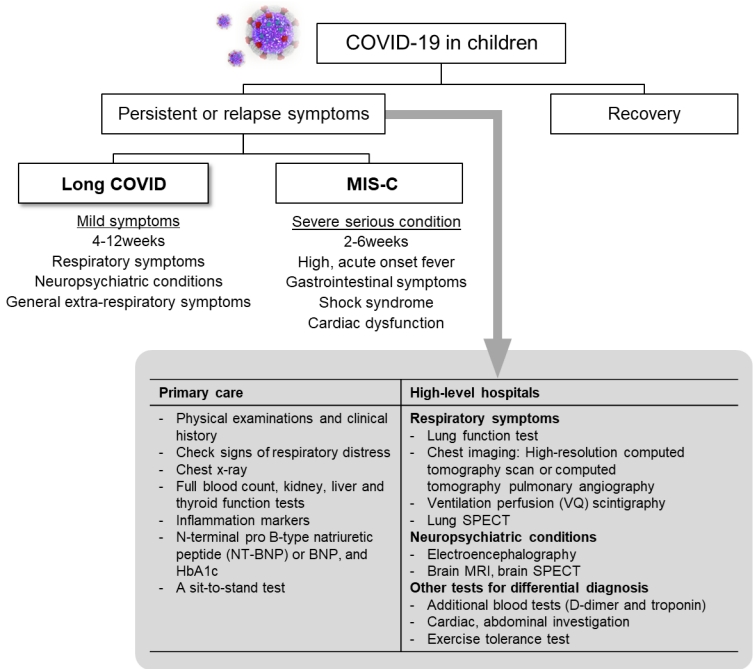
Table 1.
Recognition of long COVID in Children by Health organizations
| Organization | Terms | Duration of symptoms | Criteria |
|---|---|---|---|
| National Institute of Health and Care Excellence [14] | Long COVID | 4 Weeks or more | After acute COVID-19 |
| Persistence of symptoms | |||
| Center for Disease Control and Prevention [12] | Post COVID condition | Over 4 weeks | After SARS-CoV-2 infection |
| Physical and mental health consequences present | |||
| World Health Organization [5] | Post COVID condition | Minimum duration of 12 week | History of confirmed SARS-CoV-2 infection |
| At least one persisting physical symptom | |||
| International Child Health Group and the Royal College of Paediatrics and Child Health [72] | Long COVID | At least 4-8 weeks | After SARS-CoV-2 infection confirmation |
| Illness with symptoms lasting |
Table 2.
Epidemiologic studies of health burdens after COVID-19 in children
| Author/country/published year | Study period | COVID-19 confirmed cases (controls) | Follow-up period | Main findings of persistent symptoms related to COVID− 19 |
|---|---|---|---|---|
| Kikkenborg et al., [15] Denmark, 2022 (LongCOVIDKidsDK) | January 2020–July 2021 | 2,997 (21,640) | 2 Months | At least one symptom lasting more than 2 months in 61.9% in the case group (vs. 57.0% in the control group) Odds ratio 1.22 (95% CI, 1.15–1.30). |
| COVID-19 cases had longer-lasting symptoms and sick leave, while controls had shorter-lasting symptoms and poorer quality of life. | ||||
| Stephenson et al., [35] UK, 2022 (CLoCk) | January 2021–March 2021 | 3,065 (3,739) | 3 Months | Any symptoms observed in 66.5% (vs. 53.3%in the control group); Multiple symptoms observed in 30.3% (vs. 16.2% in the control group). |
| Risk ratio of having multiple symptoms versus very low prevalence of most physical symptoms comparing those who tested positive to those who tested negative was 1.53 (95% CI, 1.35–1.70) | ||||
| Borch et al., [42] Denmark, 2022 | January 2020–March 2021 | 15,041 (15,080) | >4 Weeks | Long COVID symptoms observed in 15%–38% |
| Children aged 6–17 years in the case group reported symptoms more frequently than the control group, percent difference 0.8%). | ||||
| Concentration difficulties, headache, muscle/joint pain, cough, nausea, diarrhea, and fever observed more frequently in controls. | ||||
| Roessler et al., [30] Germany, 2022 | January 2019–December 2020 | 11,950 (11,950) | 3 Months | Incidence rate ratios for all health outcomes in cases compared to the control cohort: 1.30 (95% CI, 1.25–1.35). |
| Medical conditions documented by a physician; Incidence rates per 1,000 person-years were significantly higher in 10 health outcomes characterized by diagnosis/symptom complexes in the COVID-19 cohort | ||||
| Magnusson et al., [31] Norway, 2022 | August 2020–February 2021 | 706,885 (275, 859) | 1–6 Months | The increase in primary care visits after 1–3 months was primarily due to respiratory and general or unspecified conditions. |
| Minor increase in primary care use for up to 6 months in 1–5 years children. |
Table 3.
The frequency of persistent symptoms in pediatric patients after COVID-19
| Reported conditions | Patients with symptom (%)* | References | ||
|---|---|---|---|---|
| 1. Respiratory symptoms | 4.1–25 | Borch [42],†, Miller [24], Stephenson [35],†, Sterky [41],†, Eitner [73],† | ||
| Dyspnea | 13.8–50 | Kikkenborg [15],†, Ashkenazi-Hoffnung [18], Brackel [19], Mizrahi [74],† | ||
| Cough | 0.5–50 | Kikkenborg [15],†, Mizrahi [74],†, Say [8], Roge [39], Katsuta [32] | ||
| Nasal symptoms | 12.4–16.1 | Buonsenso [9], Roge [39] | ||
| Sore throat | 5.7–80 | Kikkenborg [15],†, Molteni [25] | ||
| Chest pain | 1.4–31.1 | Borch [42],†, Mizrahi [74],†, Stephenson [35],†, Kikkenborg [15],†, Ashkenazi-Hoffnung [18] | ||
| 2. Neuropsychiatric conditions: | ||||
| A. Neurological symptoms | ||||
| Fatigue (tiredness) | 0.7–71.1 | Borch [42],†, Stephenson [35],†, Sterky [41], Ashkenazi-Hoffnung [18], Brackel [19], Say [8], Roge [39], Buonsenso [9], Molteni [25], Osmanov [27], Radtke [22], Katsuta [32], Eitner [73], Petersen [75] | ||
| Headache | 6.9–80 | Brackel [19], Molteni [25], Smane [26], Borch [42],†, Stephenson [35],†, Roge [39], Kikkenborg [15],†, Ashkenazi-Hoffnung [18] | ||
| Confusion | 5.6–6.5 | Zavala [43],†, Stephenson [35],† | ||
| Dizziness | 3–8.9 | Borch [42],†, Stephenson [35],†, Brackel [19], Kikkenborg [15],†, Ashkenazi-Hoffnung [18] | ||
| Memory loss | 13–17.8 | Brackel [19], Ashkenazi-Hoffnung [18] | ||
| Brain fog | 2 | Brackel [19] | ||
| B. Neurosensory symptoms | ||||
| Anosmia (loss of smell) | 1.1–84 | Borch [42],†, Stephenson [35],†, Sterky [41], Molteni [25], Smane [26], Zavala [43],†, Katsuta [32], Petersen [75], Ashkenazi-Hoffnung [18] | ||
| Ageusia (loss of taste) | 1.0–16.7 | Borch [42],†, Sterky [41], Ashkenazi-Hoffnung [18], Smane [26], Katsuta [32], Petersen [75] | ||
| Eye pain | 2.8–5.9 | Stephenson [35],†, Zavala [43],† | ||
| Ear pain | 6.2 | Stephenson [35],† | ||
| Sensory problems (i.e., cold feet) | 5.6–31 | Osmanov [27], Eitner [73], Ashkenazi-Hoffnung [18] | ||
| C. Psychiatric problems | ||||
| Sadness | 4.1 | Zavala [43],† | ||
| Mood changes, swings | 7.8–25 | Roge [39], Sterky [41],†, Zavala [43],† | ||
| Anxiety | 7.8 | Zavala [43],† | ||
| Difficulty sleeping (insomnia) | 6.9–33.3 | Ashkenazi-Hoffnung [18], Osmanov [27], Buonsenso [9], Zavala [43],† | ||
| Increased need for sleep | 2 | Radtke [22] | ||
| Concentration difficulties | 2–25 | Sterky [41], Brackel [19], Buonsenso [9], Radtke [22] | ||
| 3. General extrarespiratory symptoms | ||||
| Weight loss | 2.0–18.9 | Ashkenazi-Hoffnung [18], Brackel [19] | ||
| Persistent fever | 0.8–22.2 | Brackel [19], Katsuta [32], Smane [26] | ||
| Chills | 8.8 | Stephenson [35],† | ||
| Gastrointestinal symptoms | 20.0–25 | Sterky [41],†, Ashkenazi-Hoffnung [18], Brackel [19] | ||
| Stomach ache | 33 | Brackel [19] | ||
| Arthralgia | 14.4 | Ashkenazi-Hoffnung [18] | ||
| Myalgia | 5.4–28 | Stephenson [35],†, Ashkenazi-Hoffnung [18], Brackel [19], Mizrahi [74],† | ||
| Unspecified pain | 9 | Eitner [73] | ||
| Muscle weakness | 2.4–16 | Borch [42],†, Miller [24], Eitner [73], Mizrahi [74],† | ||
| Skin irritation/lesions | 1.6–7 | Stephenson [35],†, Brackel [19] | ||
| Loss of appetite | 2 | Brackel [19] | ||
| Vasomotor complaints | 14.4 | Ashkenazi-Hoffnung [18] | ||
| Admitted to the hospital (due to long COVID) | 18 | Brackel [19] | ||
| Microhaematuria | 11.1 | Smane [26] | ||
| Palpitations | 9.3–18 | Kikkenborg [15],†,Brackel [19] | ||
Table 4.
Possible risk factors in pediatric patients after COVID-19
References
1. World Health Organization. Coronavirus disease (COVID-19) pandemic [Internet]. Geneva (Switzerland): World Health Organization; c2023 [cited 2023 Feb 7]. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019.
2. Choi SH, Choi JH, Yun KW. Therapeutics for the treatment of coronavirus disease 2019 in children and adolescents. Clin Exp Pediatr 2022;65:377–86.




3. Lee YH, Kim HH, Choe YJ. Predicting COVID-19 transmission in a student population in Seoul, South Korea, 2020-2021. Clin Exp Pediatr 2023;66:173–8.




4. Esposito S, Principi N, Azzari C, Cardinale F, Di Mauro G, Galli L, et al. Italian intersociety consensus on management of long covid in children. Ital J Pediatr 2022;48:42.




5. Stephenson T, Allin B, Nugawela MD, Rojas N, Dalrymple E, Pinto Pereira S, et al. Long COVID (post-COVID-19 condition) in children: a modified Delphi process. Arch Dis Child 2022;107:674–80.



6. Office for National Statistics. CENSUS 2021. Technical article: Updated estimates of the prevalence of postacute symptoms among people with coronavirus (COVID-19) in the UK Articles [Internet]. Office for National Statistics; 2021 [cited 2023 Feb 7]. Available from: https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsand diseases/articles/technicalarticleupdatedestimatesoftheprevalenceofpostacutesymptomsamongpeoplewithcoronaviruscovid19intheuk/previousReleases.
7. Layton BA, Kaya D, Kelly C, Williamson KJ, Alegre D, Bachhuber SM, et al. Evaluation of a wastewater-based epidemiological approach to estimate the prevalence of SARS-CoV-2 infections and the detection of viral variants in disparate Oregon communities at city and neighborhood scales. Environ Health Perspect 2022;130:67010.



8. Say D, Crawford N, McNab S, Wurzel D, Steer A, Tosif S. Postacute COVID-19 outcomes in children with mild and asymptomatic disease. Lancet Child Adolesc Health 2021;5:e22–3.



9. Buonsenso D, Munblit D, De Rose C, Sinatti D, Ricchiuto A, Carfi A, et al. Preliminary evidence on long COVID in children. Acta Paediatr 2021;110:2208–11.




10. Beharry M. Pediatric anxiety and depression in the time of COVID-19. Pediatr Ann 2022;51:e154–60.


11. Gupta M, Gupta N, Esang M. Long COVID in children and adolescents. Prim Care Companion CNS Disord 2022;24:21r03218.


12. Long COVID or Post-COVID Conditions: National Center for Immunization and Respiratory Diseases (NCIRD) of Viral Diseases, Centers for Disease Control and Prevention [Internet]. Atlanta (GA): Centers for Disease Control and Prevention; [cited 2023 Feb 7]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/long-term-effects/.
13. World Health Organization. Post COVID-19 condition (long COVID) [Internet]. Geneva (Switzerland): World Health Organization; 2022 [cited 2023 Feb 7]. Available from: https://www.who.int/europe/newsroom/fact-sheets/item/post-covid-19-condition.
14. COVID-19 rapid guideline: managing the long-term effects of COVID-19. London: National Institute for Health and Care Excellence (NICE); 2020.
15. Kikkenborg Berg S, Dam Nielsen S, Nygaard U, Bundgaard H, Palm P, Rotvig C, et al. Long COVID symptoms in SARS-CoV-2-positive adolescents and matched controls (LongCOVIDKidsDK): a national, cross-sectional study. Lancet Child Adolesc Health 2022;6:240–8.



16. World Health Organization. A clinical case definition of post COVID-19 condition by a Delphi consensus [Internet]. World Health Organization; 2021 [cited 2023 Feb 7]. Available from: https://www.who.int/publicationsi/item/WHO-2019-nCoV-Post_COVID-19_condition-Clinical_case_definition-2021.1.
17. Jin B, Lee S, Chung US. Jeopardized mental health of children and adolescents in coronavirus disease 2019 pandemic. Clin Exp Pediatr 2022;65:322–9.




18. Ashkenazi-Hoffnung L, Shmueli E, Ehrlich S, Ziv A, Bar-On O, Birk E, et al. Long COVID in Children: Observations From a Designated Pediatric Clinic. Pediatr Infect Dis J 2021;40:e509–11.


19. Brackel CLH, Lap CR, Buddingh EP, van Houten MA, van der Sande L, Langereis EJ, et al. Pediatric long COVID: an overlooked phenomenon? Pediatr Pulmonol 2021;56:2495–502.




20. Ludvigsson JF. Case report and systematic review suggest that children may experience similar long-term effects to adults after clinical COVID-19. Acta Paediatr 2021;110:914–21.




21. Zimmermann P, Pittet LF, Curtis N. How common is long COVID in children and adolescents? Pediatr Infect Dis J 2021;40:e482–7.



22. Radtke T, Ulyte A, Puhan MA, Kriemler S. Long-term symptoms after SARS-CoV-2 infection in children and adolescents. JAMA 2021;326:869–71.



23. Office for National Statistics. CENSUS 2021. Updated estimates of the prevalence of long COVID symptoms [Internet]. Office for National Statistics; 2021 [cited 2023 Feb 7]. Available from: https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/healthandlifeexpectancies/adhocs/12788updatedestimatesoftheprevalenceoflongcovidsymptoms.
24. Miller F, Nguyen DV, Navaratnam AM, Shrotri M, Kovar J, Hayward AC, et al. Prevalence and characteristics of persistent symptoms in children during the COVID-19 pandemic: evidence from a household cohort study in england and wales. Pediatr Infect Dis J 2022;41:979–84.



25. Molteni E, Sudre CH, Canas LS, Bhopal SS, Hughes RC, Antonelli M, et al. Illness duration and symptom profile in symptomatic UK school-aged children tested for SARS-CoV-2. Lancet Child Adolesc Health 2021;5:708–18.



26. Smane L, Stars I, Pucuka Z, Roge I, Pavare J. Persistent clinical features in paediatric patients after SARS-CoV-2 virological recovery: a retrospective population-based cohort study from a single centre in Latvia. BMJ Paediatr Open 2020;4:e000905.



27. Osmanov IM, Spiridonova E, Bobkova P, Gamirova A, Shikhaleva A, Andreeva M, et al. Risk factors for post-COVID-19 condition in previously hospitalised children using the ISARIC Global follow-up protocol: a prospective cohort study. Eur Respir J 2022;59:2101341.



28. Stephenson T, Shafran R, De Stavola B, Rojas N, Aiano F, AminChowdhury Z, et al. Long COVID and the mental and physical health of children and young people: national matched cohort study protocol (the CLoCk study). BMJ Open 2021;11:e052838.



29. Kikkenborg Berg S, Palm P, Nygaard U, Bundgaard H, Petersen MNS, Rosenkilde S, et al. Long COVID symptoms in SARS-CoV-2-positive children aged 0-14 years and matched controls in Denmark (Long COVIDKidsDK): a national, cross-sectional study. Lancet Child Adolesc Health 2022;6:614–23.



30. Roessler M, Tesch F, Batram M, Jacob J, Loser F, Weidinger O, et al. Post-COVID-19-associated morbidity in children, adolescents, and adults: a matched cohort study including more than 157,000 individuals with COVID-19 in Germany. PLoS Med 2022;19:e1004122.



31. Magnusson K, Skyrud KD, Suren P, Greve-Isdahl M, Stordal K, Kristoffersen DT, et al. Healthcare use in 700 000 children and adolescents for six months after covid-19: before and after register based cohort study. BMJ 2022;376:e066809.



32. Katsuta T, Aizawa Y, Shoji K, Shimizu N, Okada K, Nakano T, et al. Acute and postacute clinical characteristics of coronavirus disease 2019 in children in Japan. Pediatr Infect Dis J 2023;42:240–6.



33. Behnood SA, Shafran R, Bennett SD, Zhang AXD, O'Mahoney LL, Stephenson TJ, et al. Persistent symptoms following SARS-CoV-2 infection amongst children and young people: a meta-analysis of controlled and uncontrolled studies. J Infect 2022;84:158–70.



34. Buonsenso D, Di Gennaro L, De Rose C, Morello R, D'Ilario F, Zampino G, et al. Long-term outcomes of pediatric infections: from traditional infectious diseases to long Covid. Future Microbiol 2022;17:551–71.



35. Stephenson T, Pinto Pereira SM, Shafran R, de Stavola BL, Rojas N, McOwat K, et al. Physical and mental health 3 months after SARS-CoV-2 infection (long COVID) among adolescents in England (CLoCk): a national matched cohort study. Lancet Child Adolesc Health 2022;6:230–9.



36. Funk AL, Kuppermann N, Florin TA, Tancredi DJ, Xie J, Kim K, et al. Post-COVID-19 conditions among children 90 days after SARS-CoV-2 infection. JAMA Netw Open 2022;5:e2223253.


37. Buonsenso D, Pujol FE, Munblit D, Pata D, McFarland S, Simpson FK. Clinical characteristics, activity levels and mental health problems in children with long coronavirus disease: a survey of 510 children. Future Microbiol 2022;17:577–88.



38. Asadi-Pooya AA, Nemati H, Shahisavandi M, Akbari A, Emami A, Lotfi M, et al. Long COVID in children and adolescents. World J Pediatr 2021;17:495–9.




39. Roge I, Smane L, Kivite-Urtane A, Pucuka Z, Racko I, Klavina L, et al. Comparison of persistent symptoms after COVID-19 and other non-SARS-CoV-2 infections in children. Front Pediatr 2021;9:752385.



40. Fainardi V, Meoli A, Chiopris G, Motta M, Skenderaj K, Grandinetti R, et al. Long COVID in children and adolescents. Life (Basel) 2022;12:285.



41. Sterky E, Olsson-Akefeldt S, Hertting O, Herlenius E, Alfven T, Ryd Rinder M, et al. Persistent symptoms in Swedish children after hospitalisation due to COVID-19. Acta Paediatr 2021;110:2578–80.




42. Borch L, Holm M, Knudsen M, Ellermann-Eriksen S, Hagstroem S. Long COVID symptoms and duration in SARS-CoV-2 positive children - a nationwide cohort study. Eur J Pediatr 2022;181:1597–607.




43. Zavala M, Ireland G, Amin-Chowdhury Z, Ramsay ME, Ladhani SN. Acute and persistent symptoms in children with polymerase chain reaction (PCR)-confirmed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection compared with test-negative children in England: active, prospective, national surveillance. Clin Infect Dis 2022;75:e191–200.




44. Raveendran AV, Jayadevan R, Sashidharan S. Long COVID: an overview. Diabetes Metab Syndr 2021;15:869–75.



45. Gaebler C, Wang Z, Lorenzi JCC, Muecksch F, Finkin S, Tokuyama M, et al. Evolution of antibody immunity to SARS-CoV-2. Nature 2021;591:639–44.


46. Rodda LB, Netland J, Shehata L, Pruner KB, Morawski PA, Thouvenel CD, et al. Functional SARS-CoV-2-specific immune memory persists after mild COVID-19. Cell 2021;184:169–83.e17.



47. Danzetta ML, Amato L, Cito F, Di Giuseppe A, Morelli D, Savini G, et al. SARS-CoV-2 RNA persistence in naso-pharyngeal Swabs. Microorganisms 2020;8:1124.



48. Xu CLH, Raval M, Schnall JA, Kwong JC, Holmes NE. Duration of respiratory and gastrointestinal viral shedding in children with SARS-CoV-2: a systematic review and synthesis of data. Pediatr Infect Dis J 2020;39:e249–56.


49. Xing YH, Ni W, Wu Q, Li WJ, Li GJ, Wang WD, et al. Prolonged viral shedding in feces of pediatric patients with coronavirus disease 2019. J Microbiol Immunol Infect 2020;53:473–80.



50. Siddiqui R, Mungroo MR, Khan NA. SARS-CoV-2 invasion of the central nervous: a brief review. Hosp Pract (1995) 2021;49:157–63.



51. Buonsenso D, Piazza M, Boner AL, Bellanti JA. Long COVID: a proposed hypothesis-driven model of viral persistence for the pathophysiology of the syndrome. Allergy Asthma Proc 2022;43:187–93.



52. Yonker LM, Neilan AM, Bartsch Y, Patel AB, Regan J, Arya P, et al. Pediatric severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): clinical presentation, infectivity, and immune responses. J Pediatr 2020;227:45–52.e5.



53. Proal AD, VanElzakker MB. Long COVID or postacute sequelae of COVID-19 (PASC): an overview of biological factors that may contribute to persistent symptoms. Front Microbiol 2021;12:698169.



54. Yong SJ. Long COVID or post-COVID-19 syndrome: putative pathophysiology, risk factors, and treatments. Infect Dis (Lond) 2021;53:737–54.



55. Jaffal SM, Abbas MA. TRP channels in COVID-19 disease: potential targets for prevention and treatment. Chem Biol Interact 2021;345:109567.



56. Bousquet J, Czarlewski W, Zuberbier T, Mullol J, Blain H, Cristol JP, et al. Potential interplay between Nrf2, TRPA1, and TRPV1 in nutrients for the control of COVID-19. Int Arch Allergy Immunol 2021;182:324–38.




57. Cheng W, Zheng J. Distribution and assembly of TRP ion channels. Adv Exp Med Biol 2021;1349:111–38.


58. Liviero F, Campisi M, Mason P, Pavanello S. Transient receptor potential vanilloid subtype 1: potential role in infection, susceptibility, symptoms and treatment of COVID-19. Front Med (Lausanne) 2021;8:753819.



59. McCarthy CG, Wilczynski S, Wenceslau CF, Webb RC. A new storm on the horizon in COVID-19: Bradykinin-induced vascular complications. Vascul Pharmacol 2021;137:106826.



60. Garvin MR, Alvarez C, Miller JI, Prates ET, Walker AM, Amos BK, et al. A mechanistic model and therapeutic interventions for COVID-19 involving a RAS-mediated bradykinin storm. Elife 2020;9:e59177.




61. Tabassum A, Iqbal MS, Sultan S, Alhuthali RA, Alshubaili DI, Sayyam RS, et al. Dysregulated bradykinin: mystery in the pathogenesis of COVID-19. Mediators Inflamm 2022;2022:7423537.




62. Dey J, Alam MT, Chandra S, Gupta J, Ray U, Srivastava AK, et al. Neuroinvasion of SARS-CoV-2 may play a role in the breakdown of the respiratory center of the brain. J Med Virol 2021;93:1296–303.



63. Solomon IH, Normandin E, Bhattacharyya S, Mukerji SS, Keller K, Ali AS, et al. Neuropathological features of Covid-19. N Engl J Med 2020;383:989–92.



64. von Weyhern CH, Kaufmann I, Neff F, Kremer M. Early evidence of pronounced brain involvement in fatal COVID-19 outcomes. Lancet 2020;395:e109.



65. Yong SJ. Persistent brainstem dysfunction in long COVID: a hypothesis. ACS Chem Neurosci 2021;12:573–80.


66. Mandal S, Barnett J, Brill SE, Brown JS, Denneny EK, Hare SS, et al. 'Long COVID': a cross-sectional study of persisting symptoms, biomarker and imaging abnormalities following hospitalisation for COVID-19. Thorax 2021;76:396–8.



67. Brodin P, Casari G, Townsend L, O'Farrelly C, Tancevski I, Loffler-Ragg J, et al. Studying severe long COVID to understand post-infectious disorders beyond COVID-19. Nat Med 2022;28:879–82.

68. Guimaraes D, Pissarra R, Reis-Melo A, Guimaraes H. Multisystem inflammatory syndrome in children (MISC): a systematic review. Int J Clin Pract 2021;75:e14450.

69. Nalbandian A, Sehgal K, Gupta A, Madhavan MV, McGroder C, Stevens JS, et al. Postacute COVID-19 syndrome. Nat Med 2021;27:601–15.


70. Ozturk GK, Beken B, Dogan S, Akar HH. Pulmonary function tests in the follow-up of children with COVID-19. Eur J Pediatr 2022;181:2839–47.




71. Daines L, Zheng B, Pfeffer P, Hurst JR, Sheikh A. A clinical review of long COVID with a focus on the respiratory system. Curr Opin Pulm Med 2022;28:174–9.



72. RCPCH responds to new study on long lasting symptoms in children with COVID-19 [Intetnet]. London: Royal College of Paediatrics and Child Health; 2021 [cited 2023 Feb 7]. Available from: https://www.rcpch.ac.uk/news-events/news/rcpch-responds-new-study-long-lastingsymptoms-children-covid-19.
73. Eitner L, Maier C, Brinkmann F, Schlegtendal A, Knoke L, EnaxKrumova E, et al. Somatosensory abnormalities after infection with SARS-CoV-2 - A prospective case-control study in children and adolescents. Front Pediatr 2022;10:977827.








 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link PubMed
PubMed Download Citation
Download Citation


