Article Contents
| Clin Exp Pediatr > Volume 67(8); 2024 |
|
Abstract
Footnotes
Conflicts of interest
YHJ has no conflicts of interest to declare. EHK reports advisory board membership with ALK, Kenota Health, and Ukko Inc; consultancy with AllerGenis, Belhaven Biopharma, Genentech, Nutricia, Revolo; and grants to his university from National Institutes of Health (NIH) and Food Allergy Research and Education (FARE)
Fig. 1.
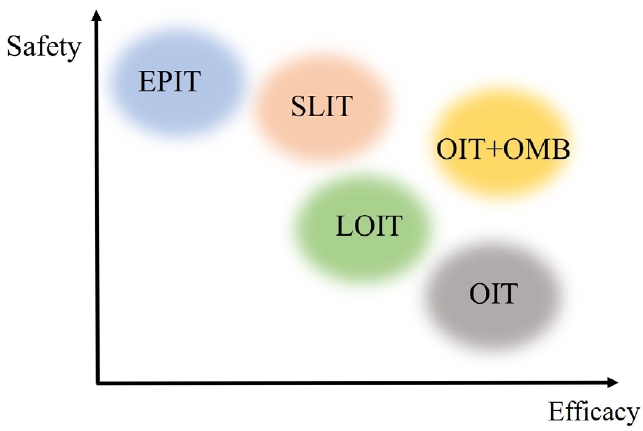
Table 1.
| Allergen/Study | Age (yr) | Numbers by group | Maintenance dose, procedure | Duration | Efficacy | Safety |
|---|---|---|---|---|---|---|
| Peanut | ||||||
| Vickery et al., 2018 [16] (PALISADE) | 4–55 | (4–17 yr) | 300 mg of peanut protein | 1 Yr | (4-17 yr) | Any AE/Severe AE |
| OIT: N=372 | Desensitization to ≥600 mg | OIT: 98.7%/4.3% | ||||
| Placebo: N=124 | OIT: 67.2% | Placebo: 95.2%/0.8% | ||||
| (18–55 yr) | Placebo: 4.0% | |||||
| OIT: N=42 | (18-55 yr) No effect | |||||
| Placebo: N=14 | ||||||
| O'B Hourihane et al., 2020 [17] (ARTEMIS) | 4–17 | OIT: N=132 | 300 mg of peanut protein | 9 Mo | Desensitized to 1,000 mg | Any AE/Severe AE |
| Placebo: N=43 | OIT: 58% | OIT: 99%/0% | ||||
| Placebo: 2% | Placebo: 98%/0% | |||||
| Vickery et al., 2021 [18] (ARC004; PALISADE follow-on study) | 4–17 | Daily dosing | 300 mg of peanut protein, Daily dosing PTAH (AR101) vs. nondaily dosing PTAH | 28–56 Wk | Desensitized to 2,000 mg | Exposure-adjusted AE/participant-year |
| PTAH Naive: N=72 | Daily dosing | Daily dosing | ||||
| Cohort 1: N=103 | PTAH Naive: 51.4% | 12.94 to 17.54 | ||||
| Cohort 3A: N=26 | Cohort 1: 48.5% | Nondaily dosing | ||||
| Nondaily dosing | Cohort 3A: 80.8% | 25.95 to 42.49 | ||||
| Cohort 2: N=38 | Nondaily dosing | |||||
| Cohort 3B: N=32 | Cohort 2: 36.8% | |||||
| Cohort 3C: N=21 | Cohort 3B: 45.4% | |||||
| Cohort 3C: 42.9% | ||||||
| Fernandez-Rivas et al., 2022 [19] (PALISADE-ARC004 post hoc longitudinal exploratory analysis) | 4–17 | Group A (28-wk maintenance): N=110 | 300 mg of peanut protein, 28-wk vs. 56-wk maintenance | 2 Yr | Desensitized to 2,000 mg | Exposure-adjusted AE (PALISADE, ARC004) |
| Group B (56-wk maintenance): N=32 | Group A: 48.1% | Group A: 20.7%, 12.8% | ||||
| Group B: 80.8% | Group B: 31.8%, 17.5% | |||||
| Jones et al., 2022 [20] (IMPACT Trial) | 1–3 | OIT: N=96 | 2,000 mg of peanut protein | 134 Wk | Desensitized to 5,000 mg | Any AE/ Severe AE |
| Placebo: N=50 | OIT group: 71% | OIT: 98%/5% | ||||
| Placebo group: 2% | Placebo: 80%/0% | |||||
| Remission: 21% vs 2% | ||||||
| Cow’s milk | ||||||
| Ogura et al., 2020 [22] | 3–15 | Low-dose OIT: N=13 | Low dose: 850 mg | 1 Yr | StU (2 wk) to 3,400 mg | Any AE per dose |
| High-dose OIT: N=13 | High-dose: 3,400 mg of CMP | Low-dose OIT: 15.4% | Low-dose OIT: 10.3% | |||
| High-dose OIT: 7.7% | High-dose OIT: 15.4$ | |||||
| Maeda et al., 2021 [21] (ORIMA) | 3–12 | OIT: N=14 | 100 mL of cow’s milk vs. avoidance | 1 Yr | Negative OFC (100 mL) | Any AE/AE requiring epinephrine |
| Control: N=14 | OIT: 50% | OIT: 86%/43% | ||||
| Control: 0% | Control: 21%/0% | |||||
| Miura et al., 2021 [23] | 5–9 | Low-dose OIT: N=36 | 3 mL of cow’s milk, 1 yr vs. 2 yr vs. 3yr | 3 Yr | 25 mL StU (2 wk) | Anaphylaxis |
| Control: N=16 | LOIT: 1 yr 27% | Hospital phase: 2% | ||||
| 2 yr 52% | Home phase: 0.04% | |||||
| 3 yr 61% | ||||||
| Control: 13% | ||||||
| Nagakura et al., 2021 [24] | 5–11 | HM-OIT: N=17 | 3 mL of cow’s milk, Heated milk vs. unheated milk | 1 Yr | Desensitized to 3 mL/ 25 mL | Mild/moderate/severe AE per home dose |
| UM-OIT: N=16 | HM-OIT: 35%/18% | HM-OIT : 7.4%/0.7%/0.02% | ||||
| UM-OIT: 50%/31% | UM-OIT: 8.1%/1.4%/0.0% | |||||
| Ibáñez-Sandín et al., 2021 [26] | 6.3–13.2 | N=58 | Omalizumab + ≥6,000 mg of CMP, omalizumab: 4 mo before OIT: omalizumab discontinued vs. continued | 1 Yr | Desensitization to ≥6,000 mg of CMP | Any AE/anaphylaxis |
| OMB discontinued: N=22 | OMB: 34.8% | OMB discontinued: 59.1%/36.4% | ||||
| OMB continued: N=16 | OMB-OIT: 83.0% | OMB continued: 25.1%/ 0% | ||||
| Dantzer et al., 2022 [25] | 3–18 | BMOIT: N=15 | 2,000 mg of BMP | 1 Yr | Desensitization to 4,044 mg of BMP | Any AE/AE requiring epinephrine |
| Placebo: N=15 | BMOIT: 73% | BMOIT: 42%/0.1% | ||||
| Placebo: 0% | Placebo: 2%/0% | |||||
| Egg | ||||||
| Kim et al., 2020 [27] | 3–16 | BE-R: N=27 | 2,000 mg of egg white protein | 2 Yr | SU (8-10 wk) to at least 4,444 mg of egg white protein | Any AE |
| OIT-R: N=23 | BE-R: 11.1% | BE-R: 2.8% | ||||
| OIT-A: N=39 | OIT-R: 43.5% | OIT-R: 3.9% | ||||
| OIT-A: 17.9% | OIT-A: 12.6% | |||||
| Ogura et al., 2020 [22] | 3–15 | Low-dose OIT: N=25 | Low dose: 1,550 mg | 1 Yr | StU (2 wk) to 6,200 mg of whole egg protein | Any AE |
| High-dose OIT: N=26 | High dose: 6,200 mg of whole egg protein | Low-dose OIT: 20% | Low-dose OIT: 8.74% | |||
| High-dose OIT: 26.9% | High-dose OIT: 10.9% | |||||
| Palosuo et al., 2021 [28] | 6–17 | OIT: N=32 | 1,000 mg of egg white protein vs avoidance, 8 mo vs. 18 mo of OIT | 8 Mo and 18 mo | Desensitization to 1,000 mg of egg white protein | Any AE in build-up phase: 82% |
| Control: N=18 | 8 mo of OIT: 44% | Severe AE: 0% | ||||
| 18 mo of OIT: 72% | ||||||
| Control: 4.8% | ||||||
| Multifood | ||||||
| Sindher, 2022 [43] | 2–25 | Low-dose mOIT: N=30 | Low dose: 300 mg | 18 Wk | IgG4/IgE ratio increase from baseline ≥25% for at least 2 allergens | Any AE/treated AE |
| High-dose mOIT: N=30 | High dose: 1,200 mg of total protein (2–5 allergens), pre-treated with 3 doses Omalizumab | Low-dose mOIT: 70% | Low-dose mOIT: 19.4%/3.6% | |||
| High-dose mOIT: 70% | High-dose mOIT: 16.8%/3.9% |
OIT, oral immunotherapy; AE, adverse event; PTAH, peanut allergen powder; StU, short-term unresponsiveness; OFC, oral food challenge; LOIT, low-dose OIT; HM-OIT, heated milk oral immunotherapy; UM-OIT, unheated milk oral immunotherapy; OMB, omalizumab; CMP, cow’s milk protein; BMOIT, baked milk oral immunotherapy; BMP, baked milk protein; SU, sustained unresponsiveness; OMB, omalizumab; BE-R, baked egg-randomized; OIT-R, egg OIT-randomized; OIT-A, egg OIT-assigned; mOIT, multifood oral immunotherapy.
Table 2.
| Study | Age (yr) | Numbers by group | Maintenance dose, procedure | Duration | Efficacy | Safety |
|---|---|---|---|---|---|---|
| SLIT | ||||||
| Fleischer et al., 2013 [30] | 12–37 | N=40 | 1,386 µg of peanut protein SLIT vs. placebo SLIT | 44 Wk and 68 wk | Passing a 5,000 mg OFC or at least ≥10 fold peanut powder than the baseline | AE beyond the oropharynx of all doses: 4.8% |
| Peanut SLIT: 70% | ||||||
| Placebo SLIT: 15% | ||||||
| Median SCD: 496 mg (44 wk) : 996 mg (68 wk) | ||||||
| Kim et al., 2019 [31] | 1–11 | N=48 | 2 mg of peanut protein SLIT | 3–5 Yr | Passing a 750 mg DBPCFC: 67% | Oropharyngeal pruritis (%) of all doses taken: 3.6% |
| Passing a 5,000 mg DBPCFC: 25% | Doses requiring epinephrine: 0% | |||||
| Median SCD:1,750 mg | ||||||
| Kim et al., 2023 [32] | 1–11 | N=54 | 4 mg of peanut protein SLIT | 4 Yr | Clinically significant desensitization (SCD > 800 mg): 70% | Oropharyngeal itching and lip swelling (%) of all doses taken: 3.7% |
| Full desensitization (SCD = 5,000 mg): 36% | Skin symptoms: 0.1% | |||||
| Abdominal symptoms: 0.1% | ||||||
| Median SCD: 2,723 mg | Doses requiring epinephrine: 0% | |||||
| EPIT | ||||||
| Fleischer et al., 2019 [36] (PEPITES) | 4–11 | Peanut patch: N=238 | 250 µg of peanut protein patch | 1 Yr | Responder criterion | Any AE/anaphylaxis |
| (1) Baseline eliciting dose | Peanut patch: 95.4%/3.4% | |||||
| Placebo patch: N=118 | ≤10 mg; posttreatment eliciting dose ≥300 mg | Placebo patch: 89%/0.4% | ||||
| (2) Baseline eliciting dose | ||||||
| >10–300 mg; ≥1,000 mg | ||||||
| Responder rate | ||||||
| Peanut patch: 35.3% | ||||||
| Placebo patch: 13.6% | ||||||
| Greenhawt et al., 2023 [37] (EPITOPE) | 1–3 | Peanut patch: N=244 | 100 µg/250 µg of peanut protein patch | 1 Yr | Responder criterion | Any AE/treatment-related anaphylaxis |
| Placebo patch: N=118 | (1) Baseline eliciting dose | Peanut patch: 100%/1.6% | ||||
| ≤10 mg; posttreatment eliciting dose ≥300 mg | Placebo patch: 99.2%/0% | |||||
| (2) Baseline eliciting dose | ||||||
| >10 mg; ≥1,000 mg | ||||||
| Responder rate | ||||||
| Peanut patch: 67% | ||||||
| Placebo patch: 33.5% |





 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link PubMed
PubMed Download Citation
Download Citation


